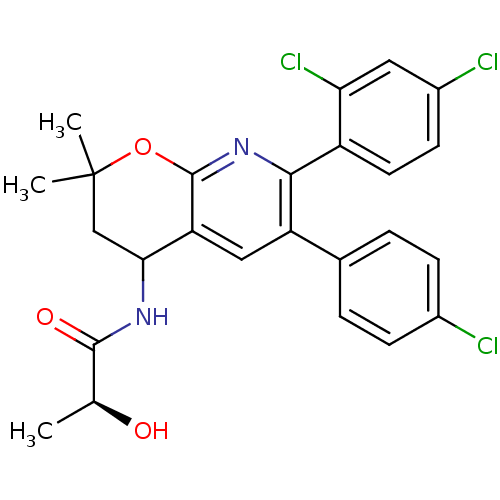
BDBM50316514 (2R)-N-[6-(4-Chlorophenyl)-7-(2,4-dichlorophenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-4-yl]-2-hydroxypropanamide::(2S)-N-(6-(4-chlorophenyl)-7-(2,4-dichlorophenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridin-4-yl)-2-hydroxypropanamide::CHEMBL1094521
SMILES C[C@H](O)C(=O)NC1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1
InChI Key InChIKey=XCFWDLWOCXSJOK-JRTLGTJJSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50316514
Found 10 hits for monomerid = 50316514
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Displacement of [3H]CP55940 from human recombinant cannabinoid CB2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Displacement of [35S]MK499 from human ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Inhibition of cannabinoid CB1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Binding affinity to human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.26E+3nMAssay Description:Binding affinity to human CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Binding affinity to human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.26E+3nMAssay Description:Binding affinity to human CB2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.26E+3nMAssay Description:Inhibition of cannabinoid CB2 receptorMore data for this Ligand-Target Pair