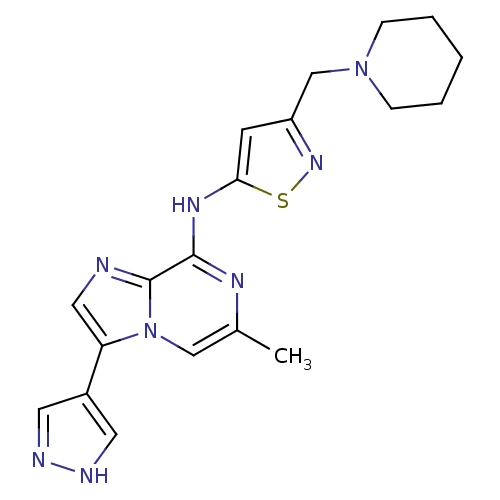
BDBM50335179 6-Methyl-N-[3-(1-piperidinylmethyl)-5-isothiazolyl]-3-(1Hpyrazol-4-yl)imidazo[1,2-a]pyrazin-8-amine Hydrochloride::CHEMBL1650536::CHEMBL1739550
SMILES Cc1cn2c(cnc2c(Nc2cc(CN3CCCCC3)ns2)n1)-c1cn[nH]c1
InChI Key InChIKey=SKDSRJRRAQLUPP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50335179
Found 10 hits for monomerid = 50335179
Affinity DataEC50: 50nMAssay Description:Inhibition of aurora B kinase in human HCT116 cells assessed as inhibition of histone H3 phosphorylation incubated for 1 hr by Hoechst 33342 staining...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of Aurora B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of BRK pretreated for 30 mins by microplate readerMore data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:Inhibition of phosphorylated SAM68 in 293 WT-PTK6 cells after 3 hrsMore data for this Ligand-Target Pair