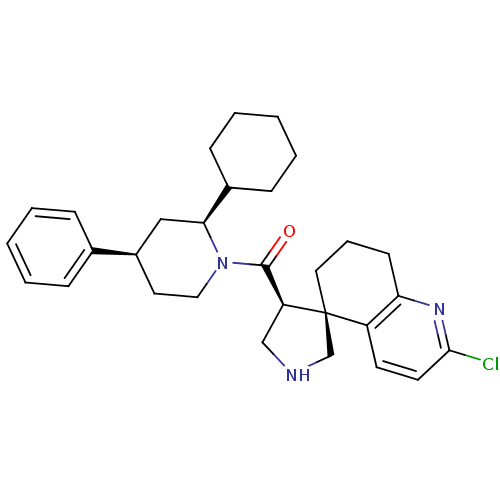
BDBM50360723 CHEMBL1934295
SMILES Clc1ccc2c(CCC[C@]22CNC[C@H]2C(=O)N2CC[C@H](C[C@H]2C2CCCCC2)c2ccccc2)n1
InChI Key InChIKey=BVBQXGDSJPQNKF-PCUPTAKISA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50360723
Found 5 hits for monomerid = 50360723
Affinity DataIC50: 570nMAssay Description:Inhibition of human BACE1 expressed in mouse fibroblast cells assessed as inhibition of soluble APPbeta_NF cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of BACE1 in cell-free systemMore data for this Ligand-Target Pair
Affinity DataIC50: 6.21E+3nMAssay Description:Inhibition of Cat DMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+5nMAssay Description:Inhibition of reninMore data for this Ligand-Target Pair