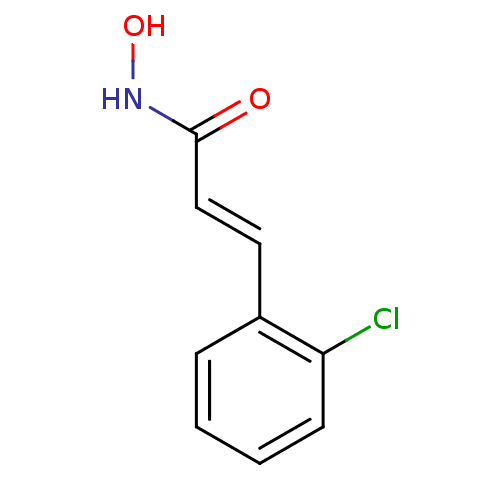
BDBM50383528 CHEMBL2032251
SMILES ONC(=O)\C=C\c1ccccc1Cl
InChI Key InChIKey=HMGPSDPNANTYKH-AATRIKPKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50383528
Found 4 hits for monomerid = 50383528
TargetBotulinum neurotoxin type A(Clostridium botulinum)
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Fox Chase Chemical Diversity Center
Curated by ChEMBL
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibition of clostridium botulinum Botulinum neurotoxin type A using SNAPide as substrate by FRET analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductaseMore data for this Ligand-Target Pair