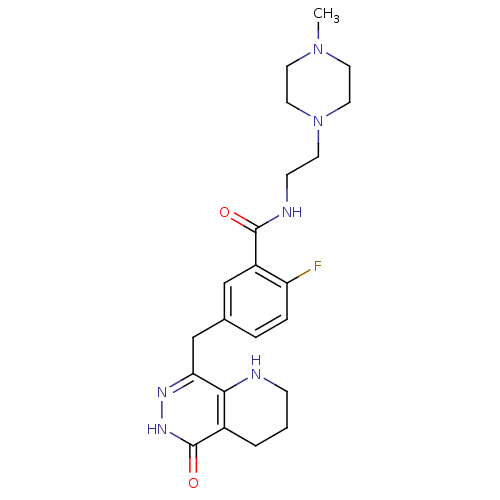
BDBM50387929 CHEMBL2058693::US9283222, 563
SMILES CN1CCN(CCNC(=O)c2cc(Cc3n[nH]c(=O)c4CCCNc34)ccc2F)CC1
InChI Key InChIKey=LTXGZFJBIQKXCW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50387929
Found 3 hits for monomerid = 50387929
Affinity DataKi: 3.5nMpH: 8.0Assay Description:PARP1 assay was conducted in PARP assay buffer containing 50 mM Tris pH 8.0, 1 mM DTT, 4 mM MgCl2. PARP reactions contained 1.5 uM [3H]-NAD+ (1.6 uCi...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Inhibition of PARP1 using [3H]NAD+ after 1 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Inhibition of PARP1 in H202-stimulated human C41 cells incubated for 30 mins prior to H2O2-treatment measured after 10 mins by FITC-based immunostain...More data for this Ligand-Target Pair