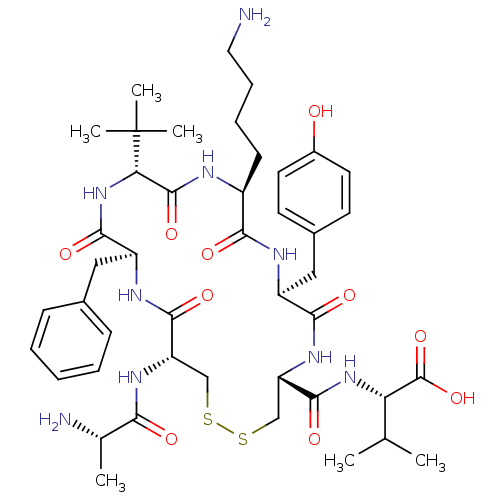
BDBM50445378 CHEMBL3104642
SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(C)(C)C)C(O)=O
InChI Key InChIKey=PHHPOPBJNOMNPB-LEZLZINTSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50445378
Found 4 hits for monomerid = 50445378
Affinity DataEC50: >0nMAssay Description:Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstrictionMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataEC50: >0nMAssay Description:Agonist activity at urotensin-2 receptor in Sprague-Dawley rat aortic rings assessed as KCl-induced vasoconstrictionMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair