BDBM50535041 CHEMBL1312860
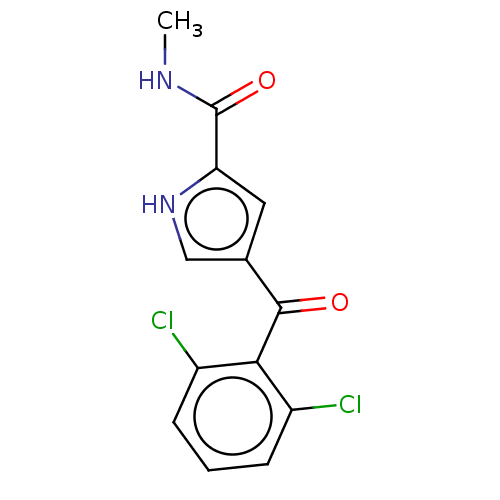
SMILES CNC(=O)c1cc(c[nH]1)C(=O)c1c(Cl)cccc1Cl
InChI Key InChIKey=IMCVPHRDALQWTJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50535041
Found 3 hits for monomerid = 50535041
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKd: 5.00E+4nMAssay Description:Binding affinity to biotinylated Avi tagged human DoT1L (2 to 416 residues) by SPR analysisMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.20E+5nMAssay Description:Inhibition of DOT1L (unknown origin)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.20E+5nMAssay Description:Inhibition of human DOTL1 (2 to 416 residues)-mediated methylation of nucleosome preincubated for 30 mins followed by addition of S-[methyl-3H-] aden...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)