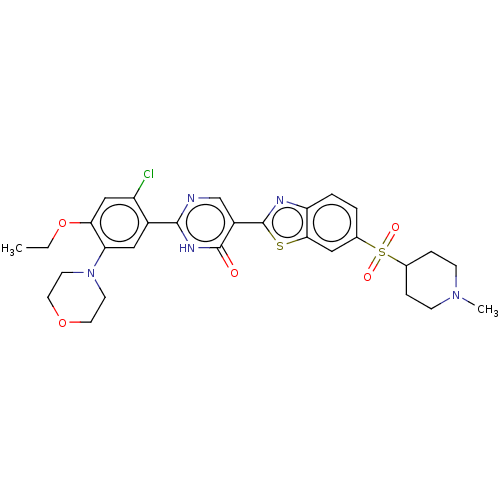
BDBM50539690 CHEMBL4645314
SMILES CCOc1cc(Cl)c(cc1N1CCOCC1)-c1ncc(-c2nc3ccc(cc3s2)S(=O)(=O)C2CCN(C)CC2)c(=O)[nH]1
InChI Key InChIKey=KGZNYDFUINOSHN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50539690
Found 3 hits for monomerid = 50539690
Affinity DataEC50: 240nMAssay Description:Agonist activity at human GPR81 overexpressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP productionMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+3nMAssay Description:Agonist activity at human GPR109A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.80E+4nMAssay Description:Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter methodMore data for this Ligand-Target Pair