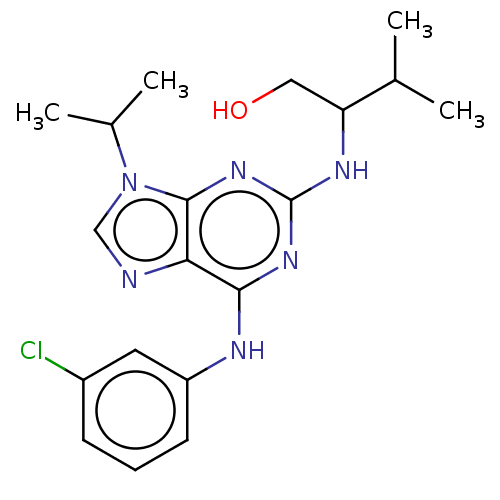
BDBM50546239 CHEMBL103579
SMILES CC(C)C(CO)Nc1nc(Nc2cccc(Cl)c2)c2ncn(C(C)C)c2n1
InChI Key InChIKey=PMXCMJLOPOFPBT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50546239
Found 3 hits for monomerid = 50546239
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 5.50E+3nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: <50nMAssay Description:Inhibition of human CDK2/cyclinA using histone H1 as substrate incubated for 30 mins in presence of gamma[32P]ATP by phosphoimaging analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 6.58E+3nMAssay Description:Inhibition of human AKR1C3 expressed in Escherichia coli incubated for 30 mins in presence of NADPH regeneration system by UHPLC analysisMore data for this Ligand-Target Pair