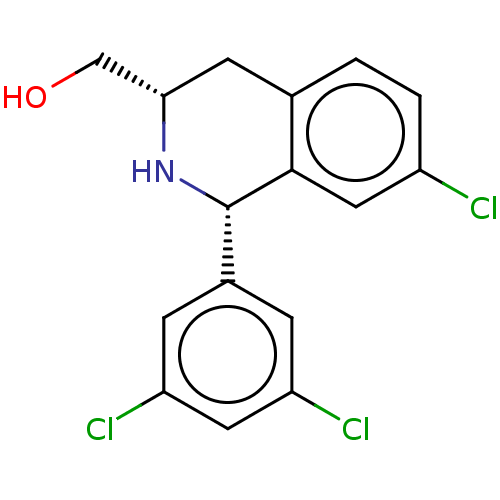
BDBM50602163 CHEMBL5189636
SMILES OC[C@@H]1Cc2ccc(Cl)cc2[C@@H](N1)c1cc(Cl)cc(Cl)c1
InChI Key InChIKey=HWDYWVRNXBUCEH-HOCLYGCPSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50602163
Found 2 hits for monomerid = 50602163
TargetTransient receptor potential cation channel subfamily M member 4(Homo sapiens)
Turning Point Therapeutics
Curated by ChEMBL
Turning Point Therapeutics
Curated by ChEMBL
Affinity DataEC50: 8.91E+3nMAssay Description:Agonist activity at human TRPM4 expressed in CHO-K1 cells assessed as increase in relative fluorescence unit by measuring membrane potential by FLIPR...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily M member 5(Homo sapiens (Human))
Turning Point Therapeutics
Curated by ChEMBL
Turning Point Therapeutics
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Agonist activity at human TRPM5 expressed in CHO-K1 cells assessed as increase in relative fluorescence unit measured for 2 mins by FLIPR based membr...More data for this Ligand-Target Pair