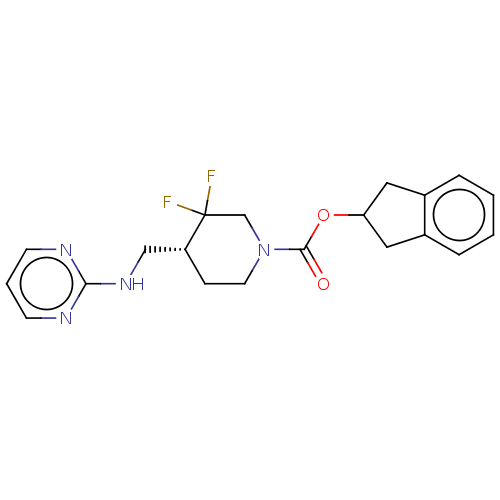
BDBM617049 US11752155, Compound III-E1-22.1B'
SMILES FC1(F)CN(CC[C@@H]1CNc1ncccn1)C(=O)OC1Cc2ccccc2C1
InChI Key InChIKey=ZRVYDDFEOLDULP-MRXNPFEDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 617049
Found 3 hits for monomerid = 617049
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Rugen Holdings (Cayman)
US Patent
Rugen Holdings (Cayman)
US Patent
Affinity DataKi: 1.5nMAssay Description:HEK293 cell lines stably expressing cloned human NR1/NR2B and NR1/NR2A, respectively, were established according to standard previously described met...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Rugen Holdings (Cayman)
US Patent
Rugen Holdings (Cayman)
US Patent
Affinity DataIC50: 3.50E+4nMAssay Description:The assay was performed on hERG channel stably expressed in HEK293 cells. The cells were cultured at 37° C. in a humidified CO2 incubator in the grow...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibitory activities of test compounds on 5 major isoforms of CYP P450 were evaluated by using pooled human liver microsome (HLM, purchased from BD ...More data for this Ligand-Target Pair