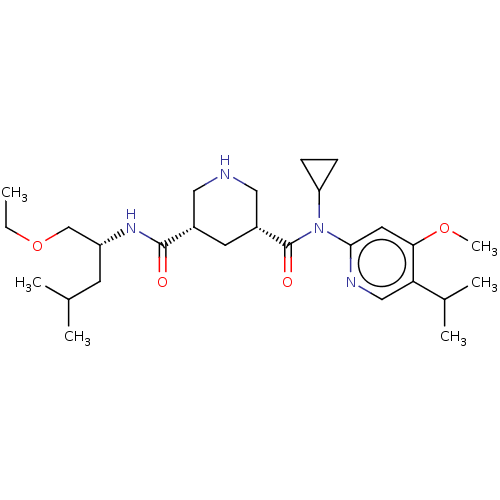
BDBM98679 US8497286, 155
SMILES CCOC[C@@H](CC(C)C)NC(=O)[C@@H]1CNC[C@@H](C1)C(=O)N(C1CC1)c1cc(OC)c(cn1)C(C)C
InChI Key InChIKey=AOJMWYPUZRQUTK-PWRODBHTSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 98679
Found 4 hits for monomerid = 98679
Affinity DataIC50: 0.900nMAssay Description:Inhibition activity of renin using FRET assay.More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERG expressed in HEK293 cell membranes incubated for 90 mins by beta-counting methodMore data for this Ligand-Target Pair