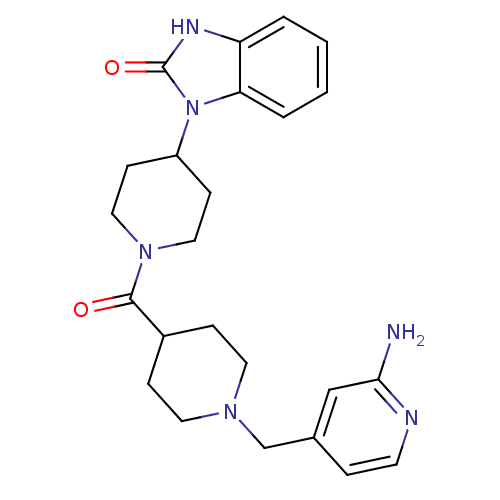
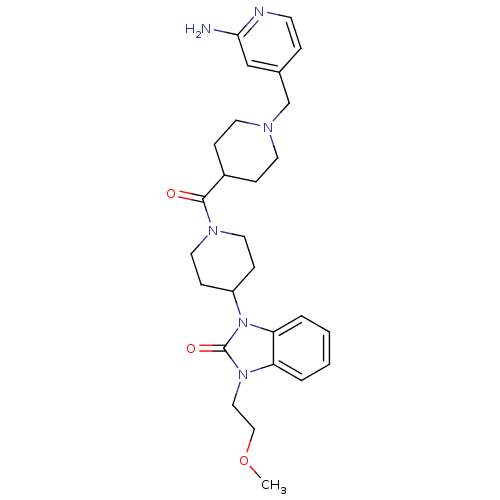
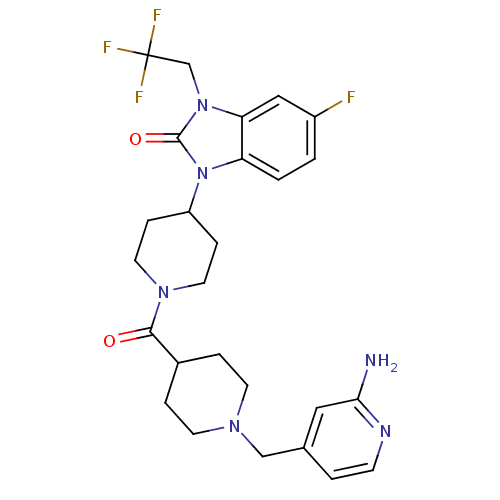
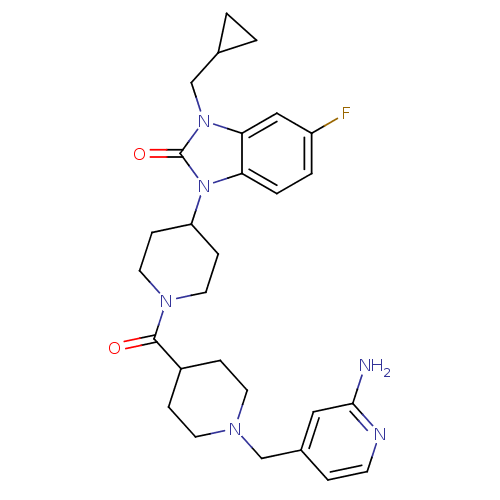
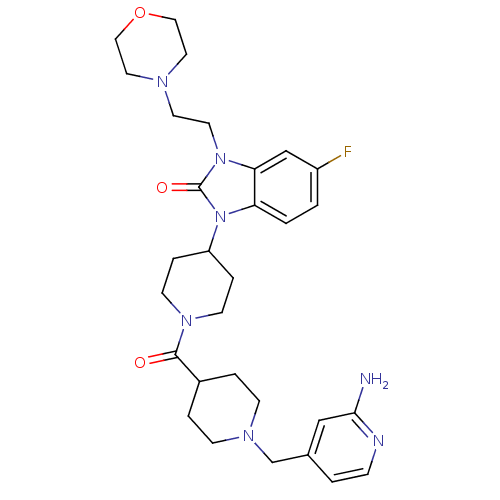
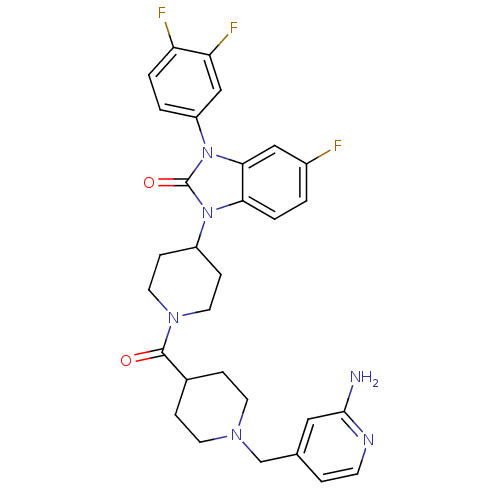
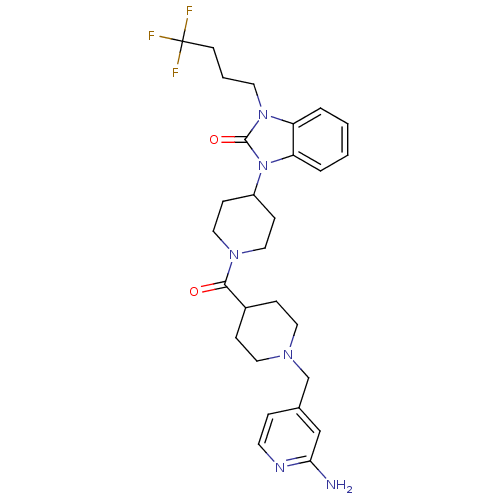
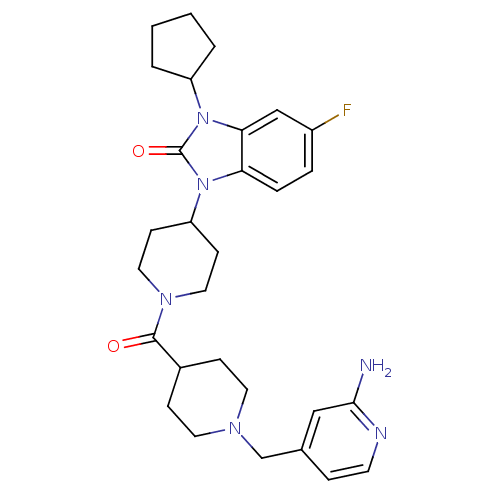
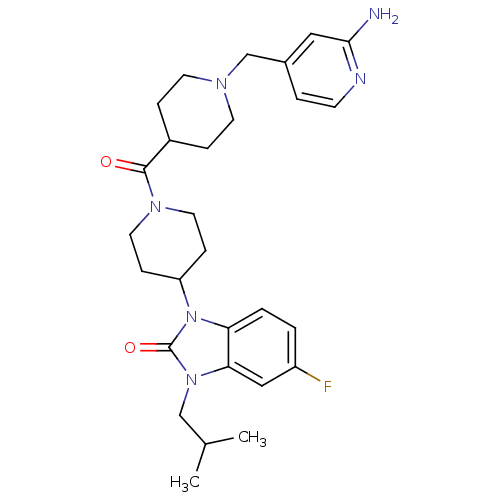
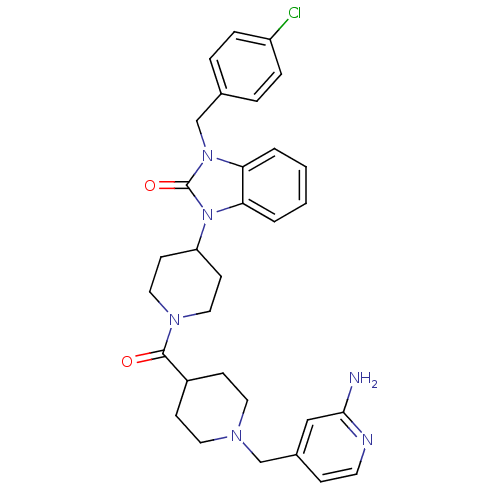
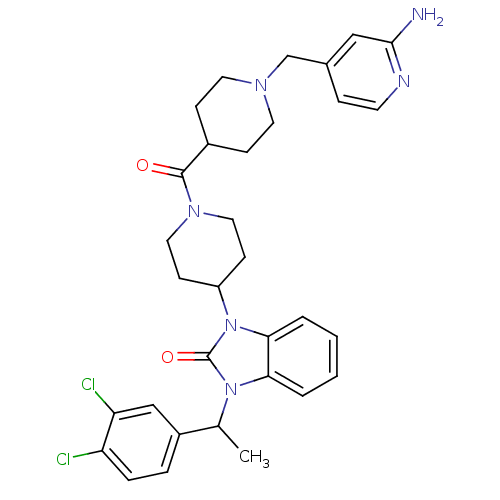
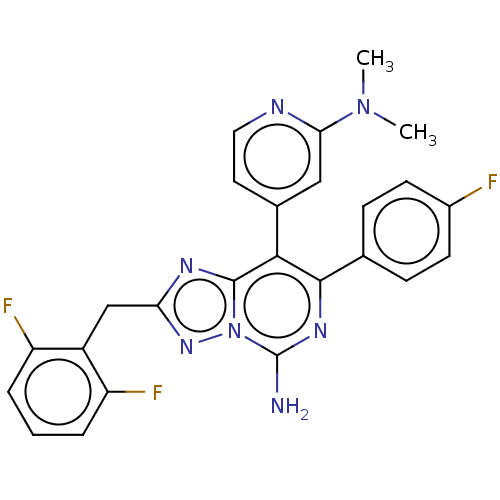
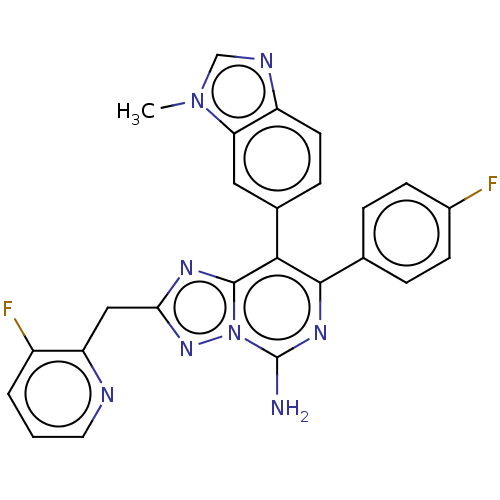
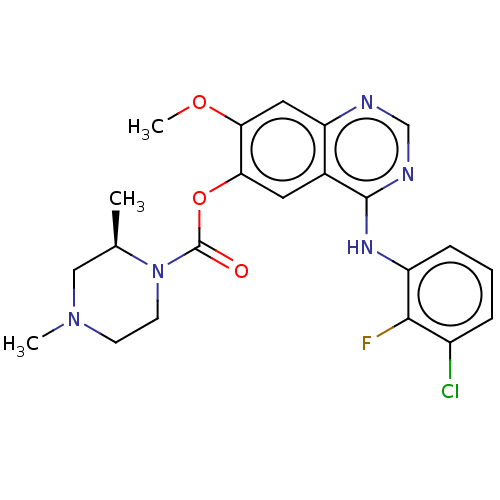
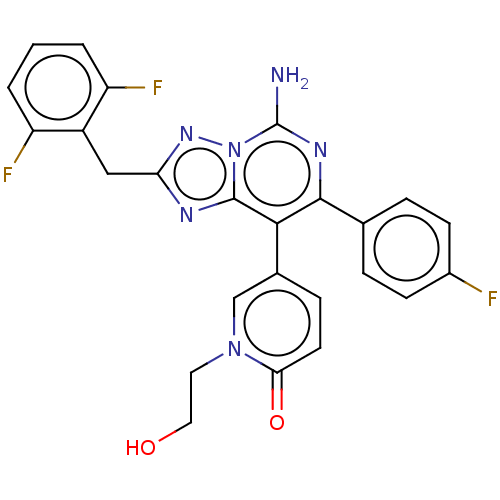
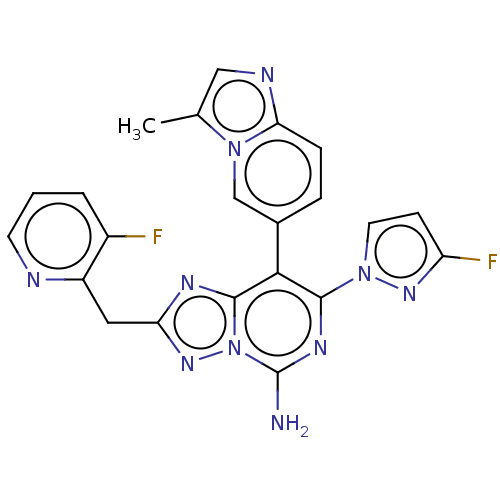
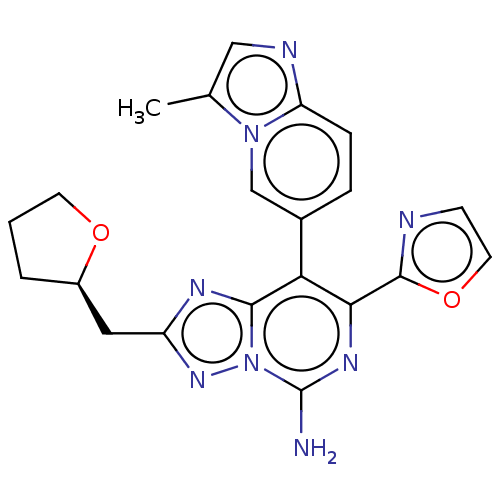
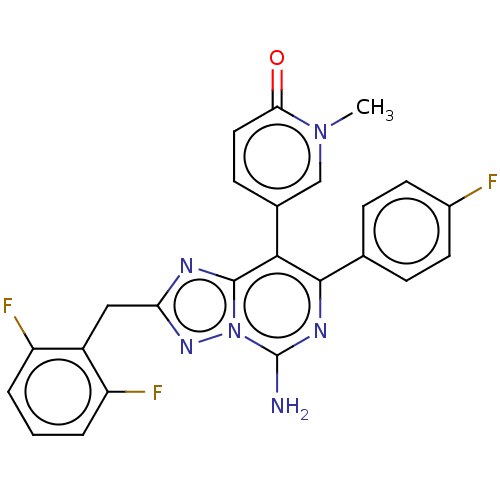
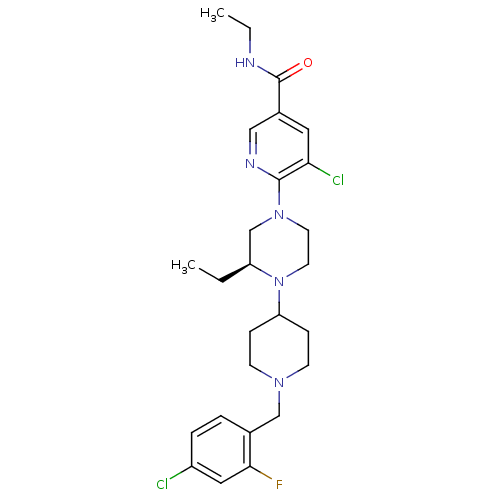
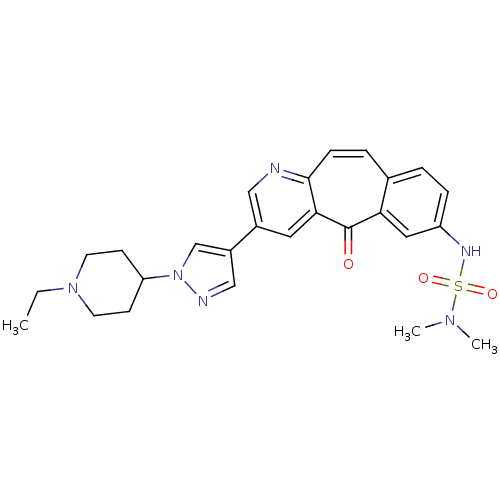
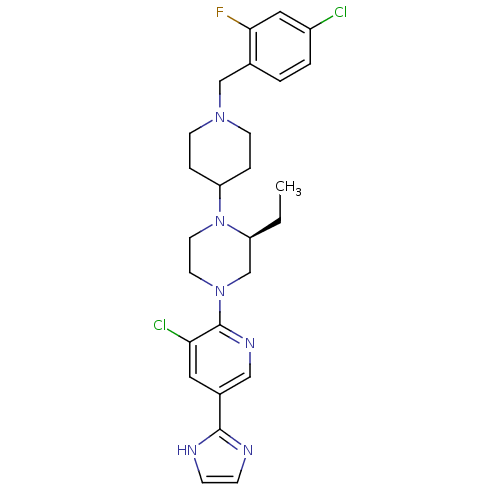
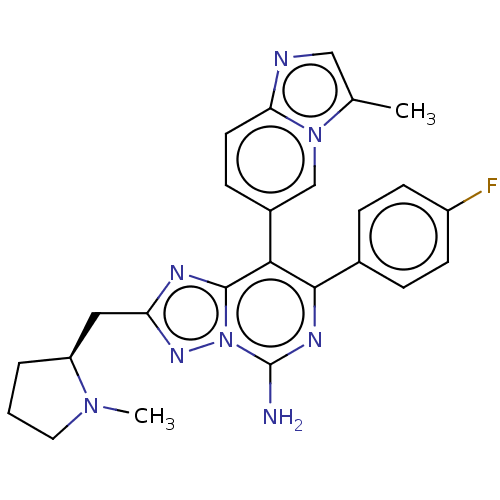
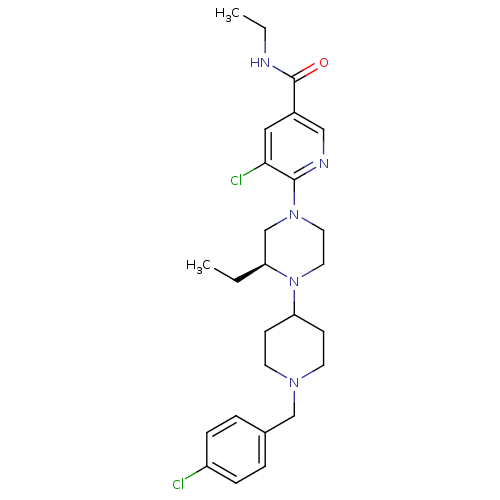
Affinity DataKi: 0.5nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 0.950nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 7.60nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 77nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenatesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Binding affinity and specificities of the compounds against different subtype of human adenosine receptors (hA1, hA2A, hA2B and hA3) were characteriz...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Binding affinity and specificities of the compounds against different subtype of human adenosine receptors (hA1, hA2A, hA2B and hA3) were characteriz...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) by HTRF assay in presence of Km of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) by HTRF assay in presence of Km of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:hADORA1/CHO (hA1 expressing) cells (Genscript) were plated at 1×104 cells/well into 384-well polystyrene plates one day before starting the experimen...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Binding affinity and specificities of the compounds against different subtype of human adenosine receptors (hA1, hA2A, hA2B and hA3) were characteriz...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Binding affinity to human CXCR3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at human CXCR3More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant c-Met-catalyzed phosphorylation of N-biotinylated peptide (EQEDEPEGDYFEWLE-CONH2) by time-resolved fluorescence reson...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Binding affinity to human CXCR3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:hADORA1/CHO (hA1 expressing) cells (Genscript) were plated at 1×104 cells/well into 384-well polystyrene plates one day before starting the experimen...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of EGFR (unknown origin) by HTRF assay in presence of Km of ATPMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at human CXCR3More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cellsMore data for this Ligand-Target Pair