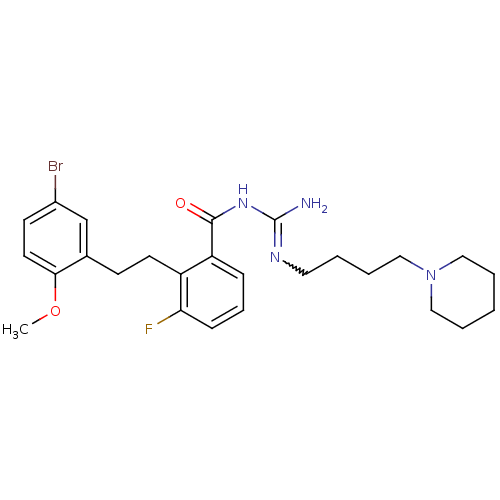
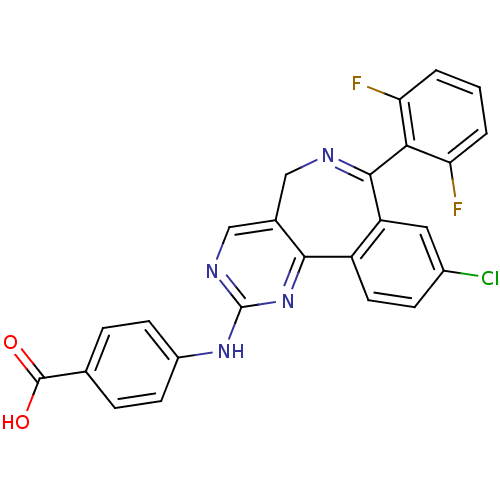
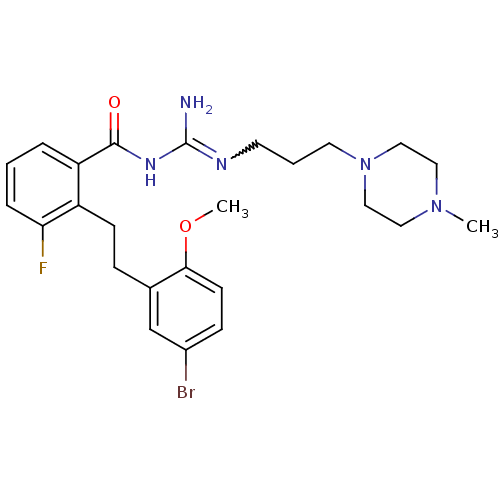
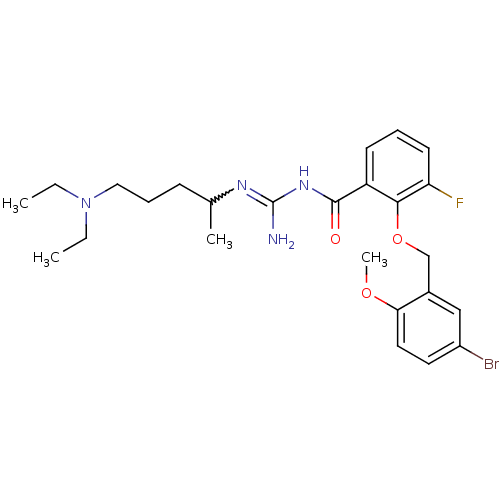
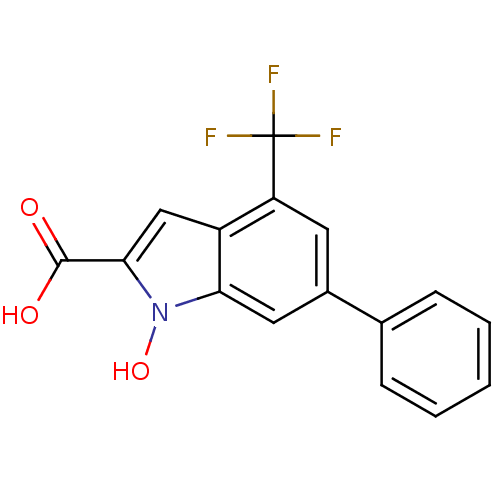
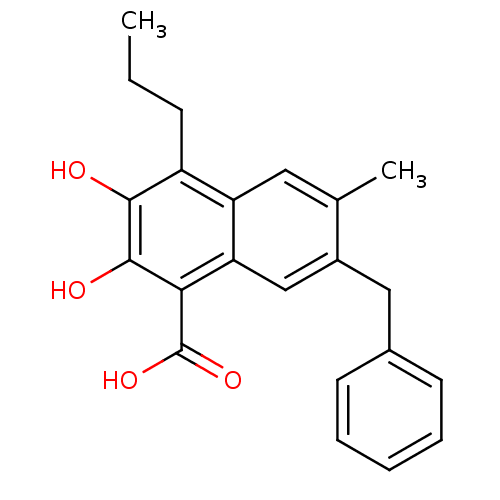
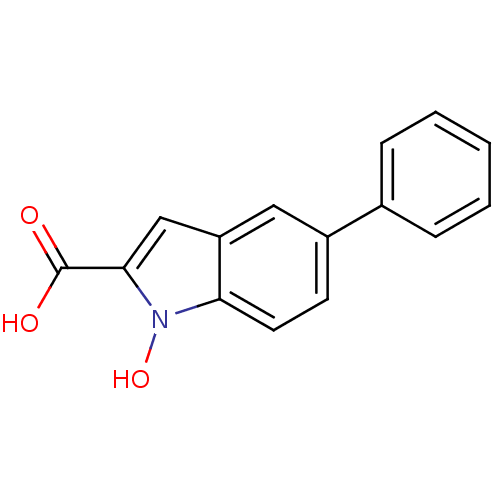
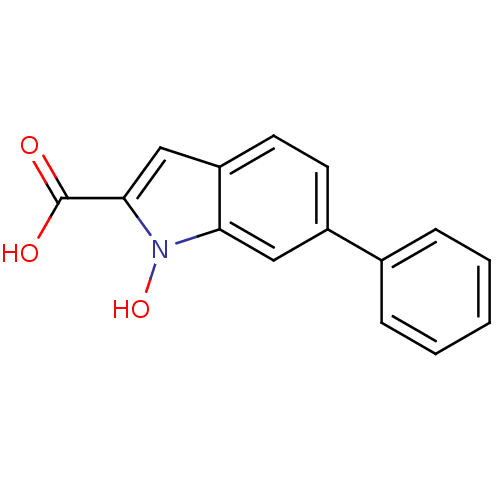
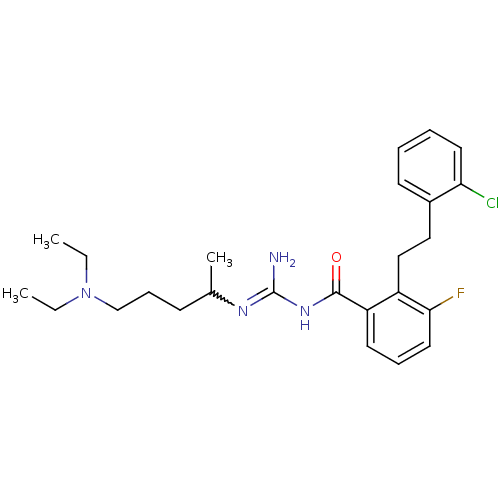
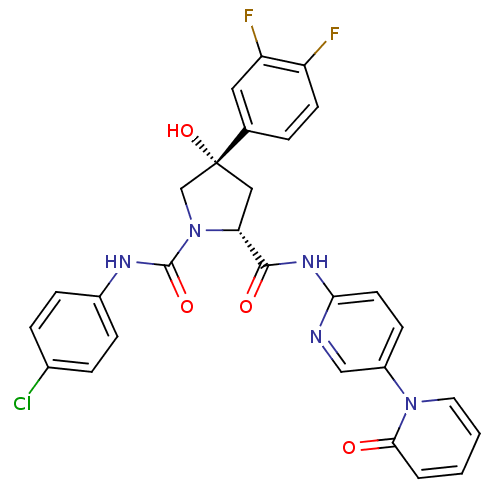
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.00200nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
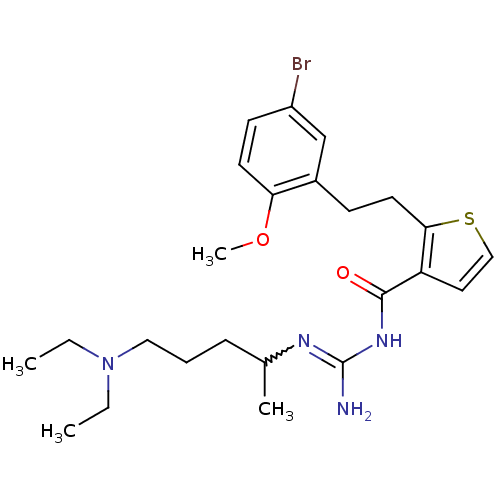
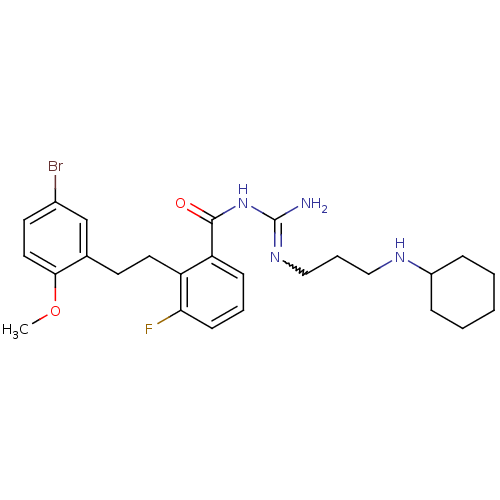
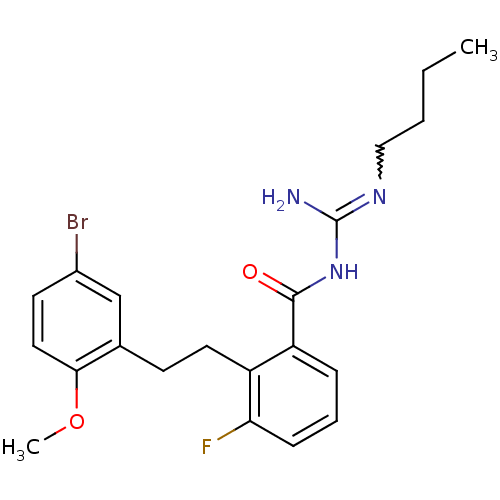
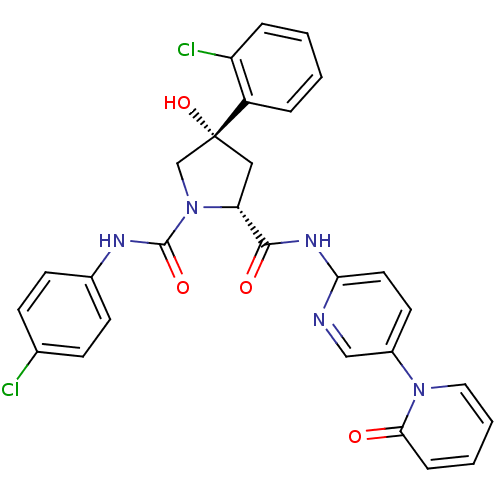
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0120nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
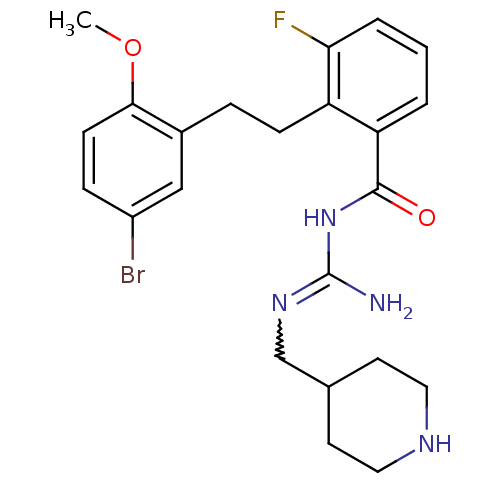
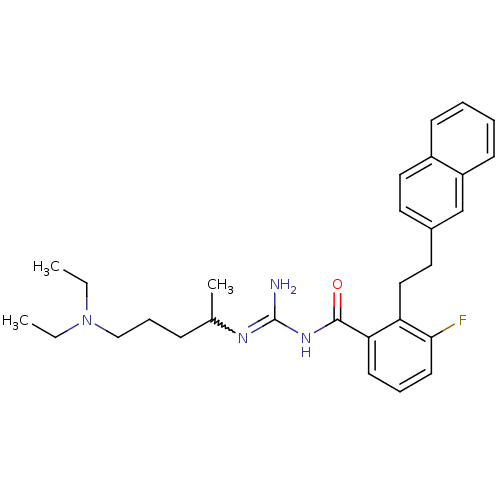
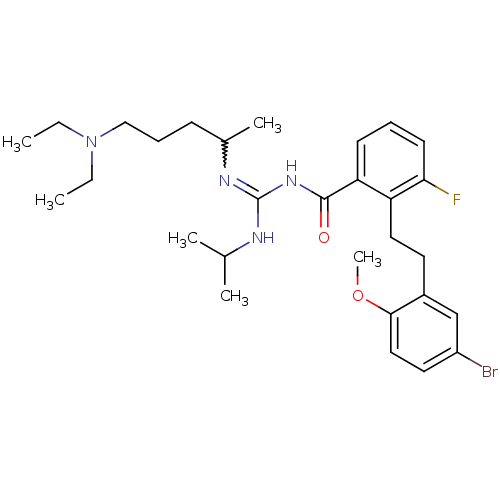
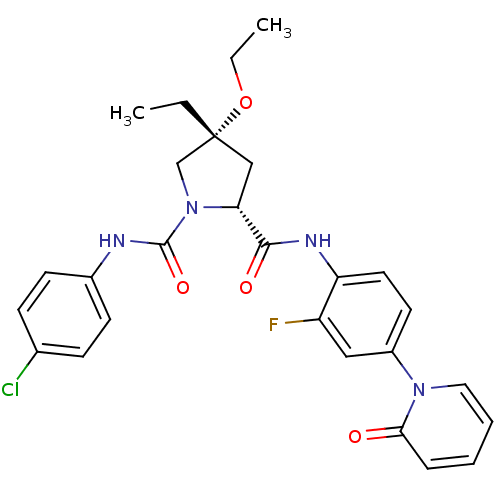
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATPMore data for this Ligand-Target Pair
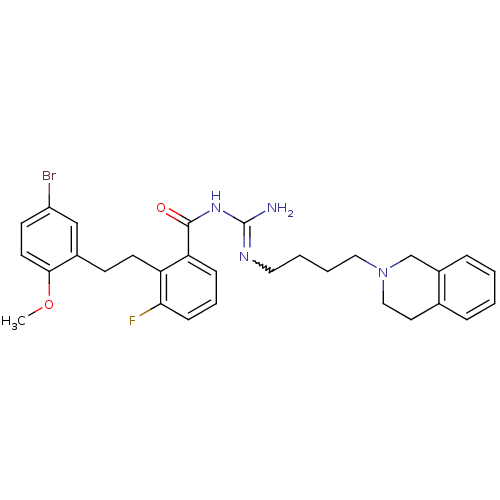
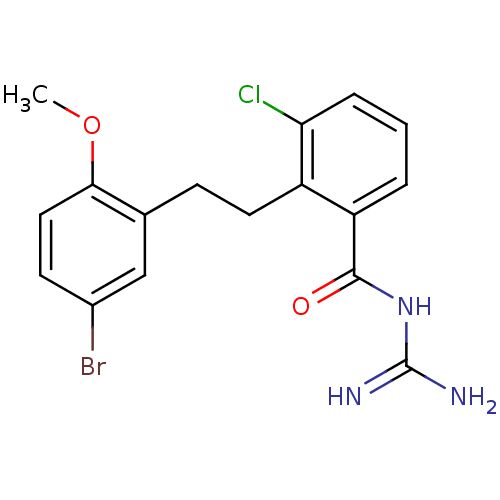
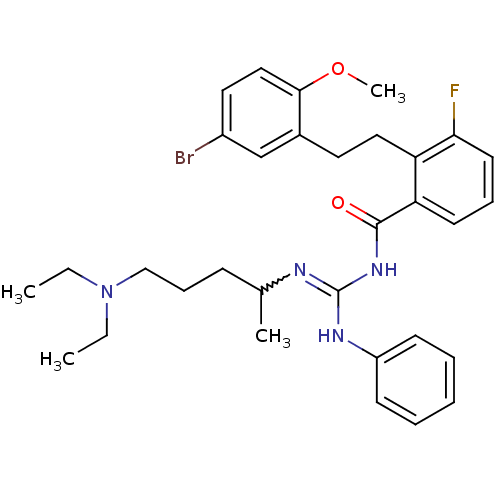
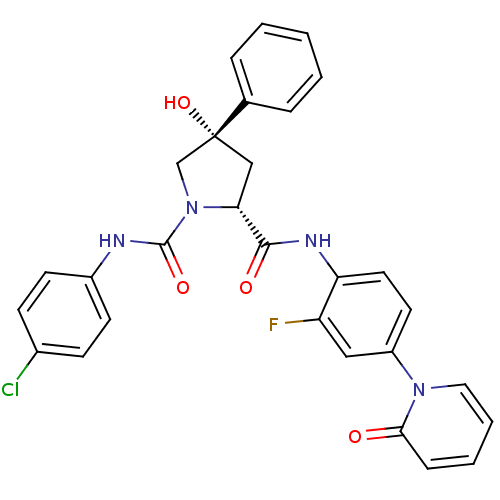
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.360nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataKi: 0.580nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Binding affinity to human Angiotensin receptor 1More data for this Ligand-Target Pair
Affinity DataKi: 0.830nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATPMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 89nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 770nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
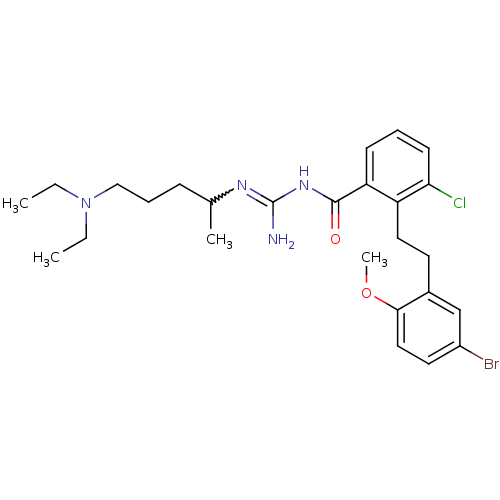
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Millennium Pharmaceuticals
Curated by ChEMBL
Millennium Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Binding affinity to hERGMore data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 920nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
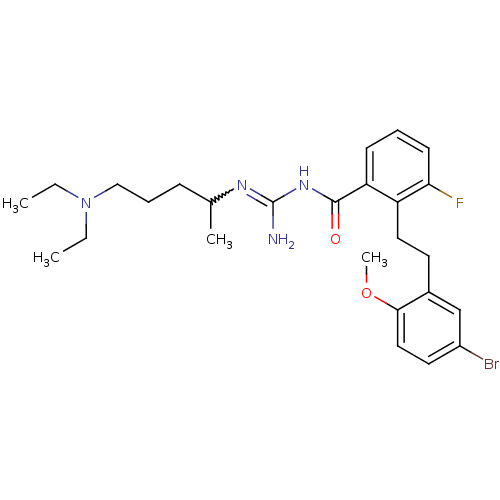
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Millennium Pharmaceuticals
Curated by ChEMBL
Millennium Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.15E+3nMAssay Description:Binding affinity to hERGMore data for this Ligand-Target Pair
Affinity DataKi: 4.10E+3nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 6.30E+3nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 8.90E+3nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human Angiotensin receptor 2More data for this Ligand-Target Pair
Affinity DataKi: 1.04E+4nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.57E+4nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+4nMAssay Description:Binding affinity to MC4R by membrane filtration assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.54E+4nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+5nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of human Factor-10aMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Inhibition of human Factor-10aMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of human Factor-10aMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of human Factor-10aMore data for this Ligand-Target Pair
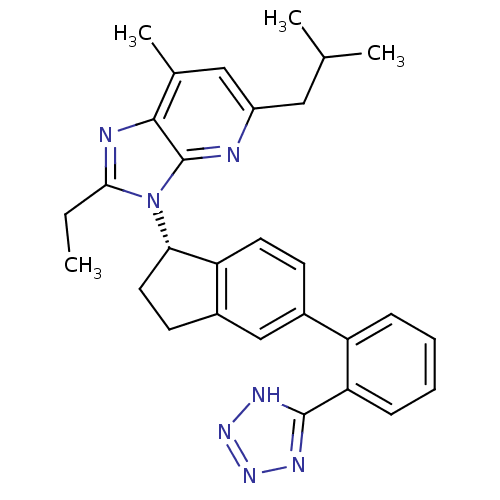
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataIC50: <0.0800nMAssay Description:Inhibition of recombinant N-terminal GST-fused LRRK2 G2109S mutant (970 to 2527 residues) (unknown origin) preincubated with enzyme for 15 mins follo...More data for this Ligand-Target Pair


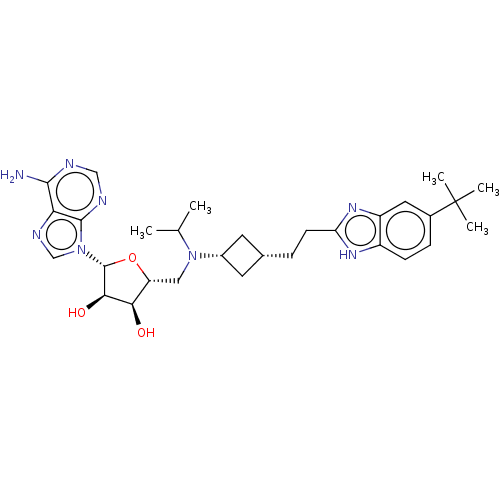
 3D Structure (crystal)
3D Structure (crystal)