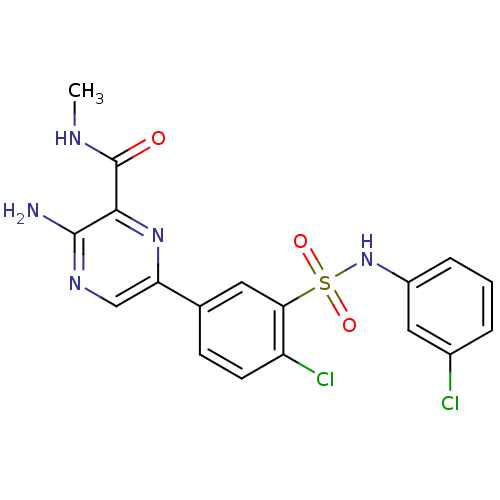
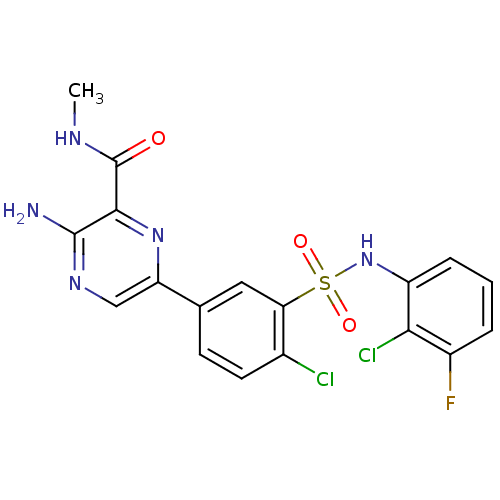
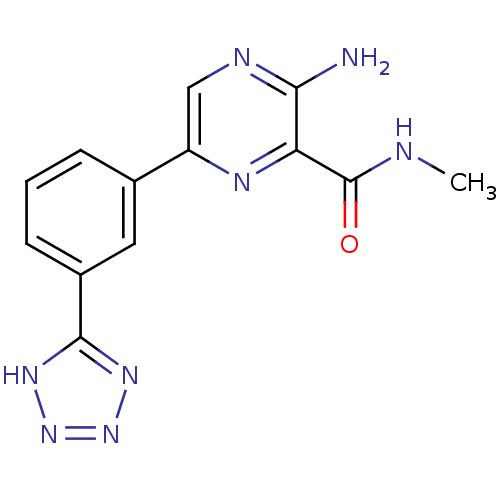
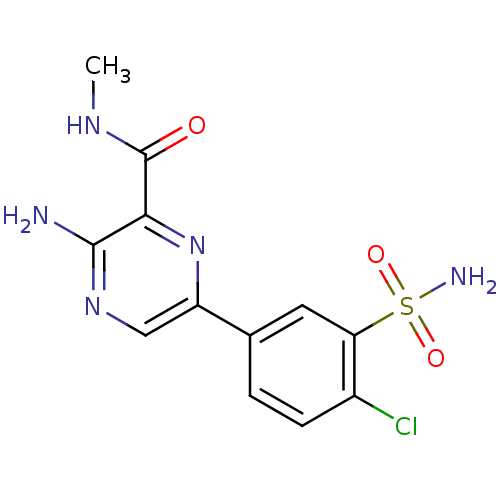
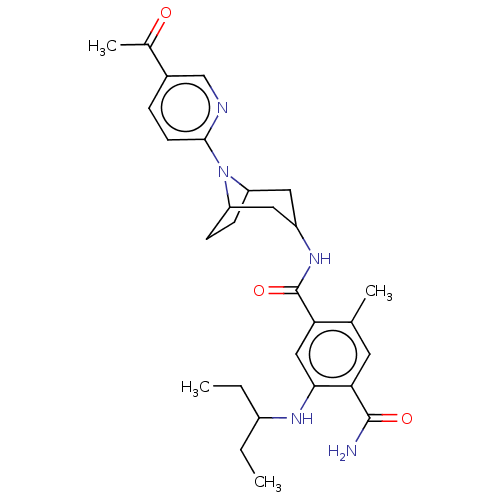
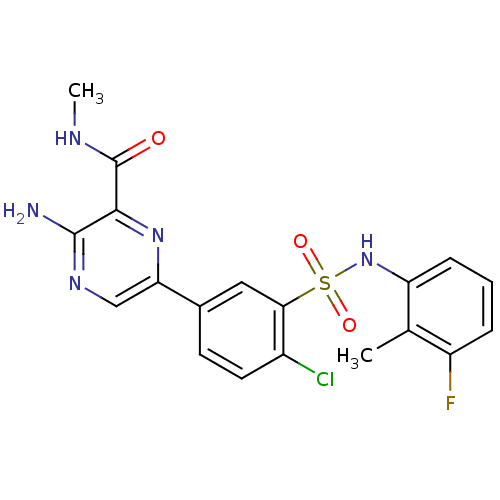
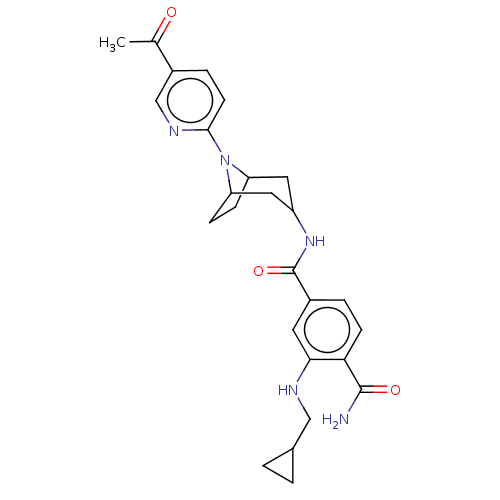
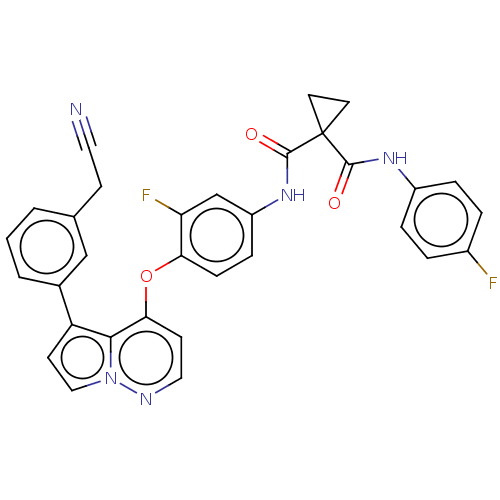
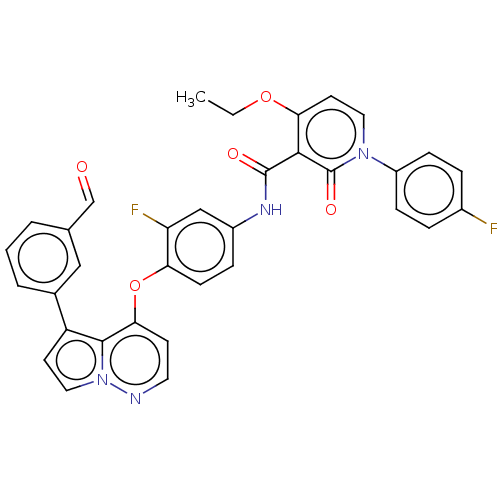
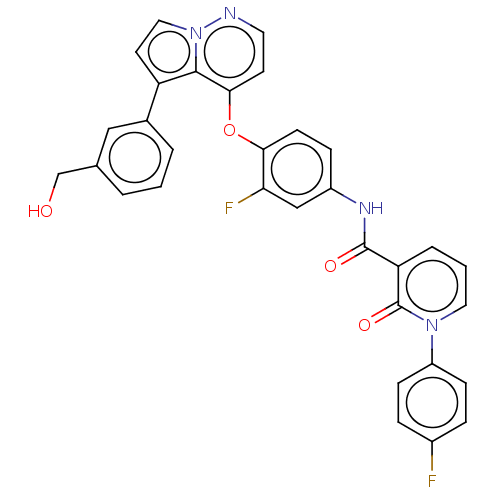
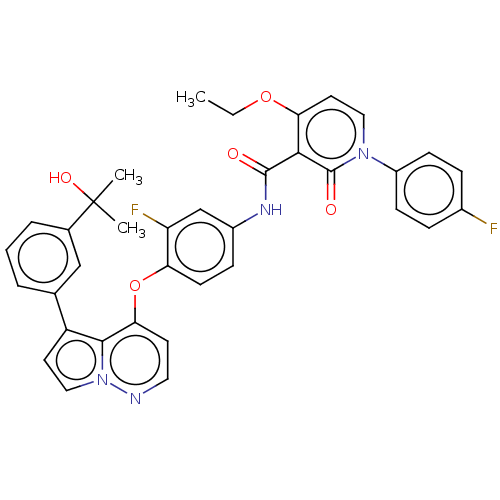
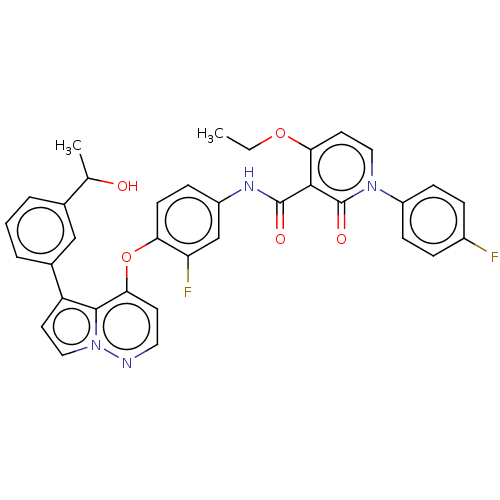
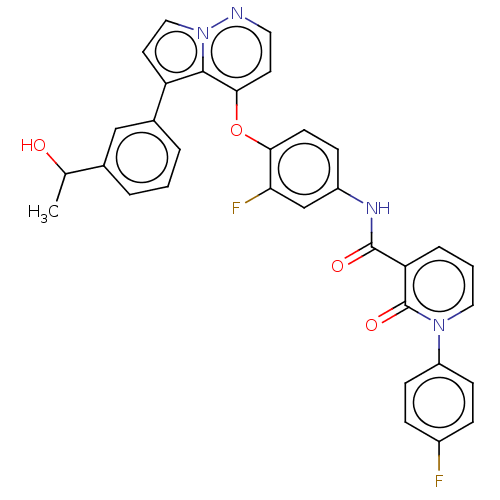
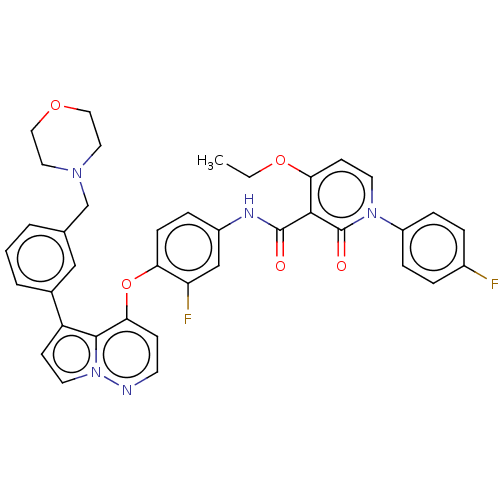
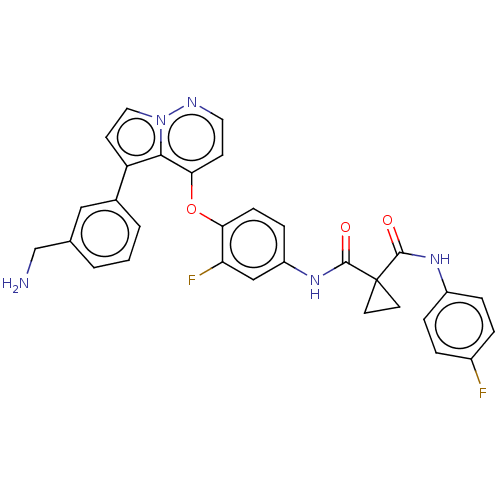
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
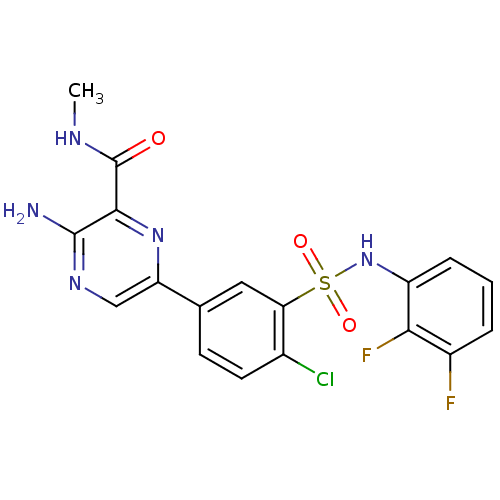
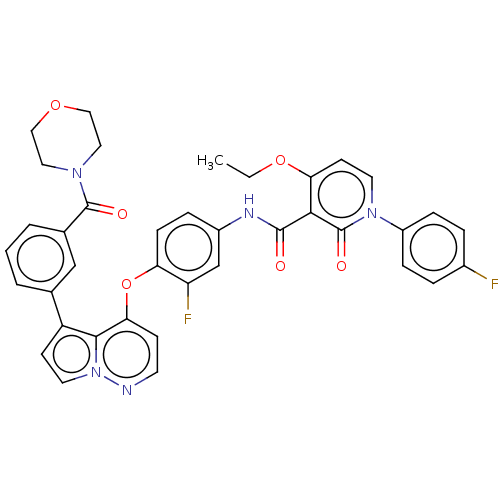
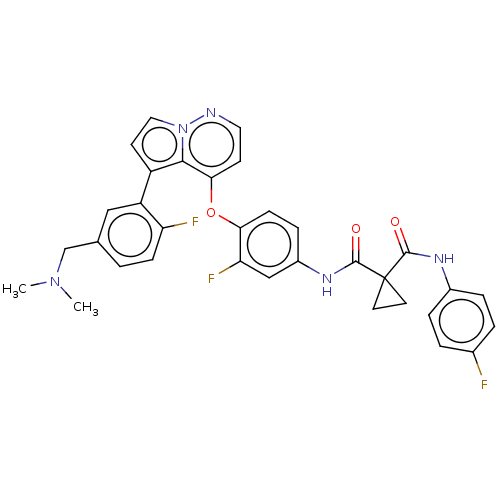
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
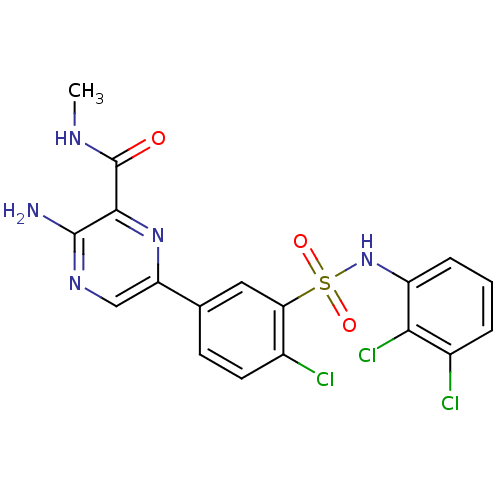
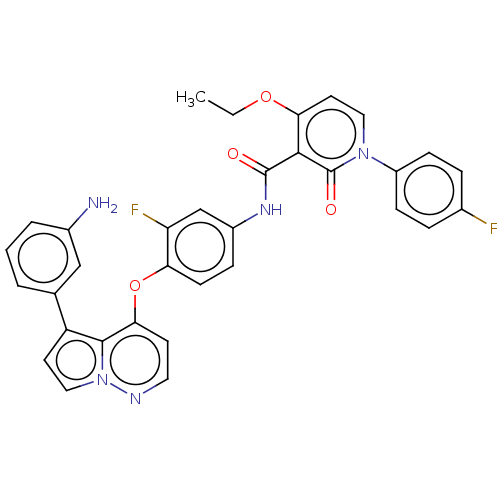
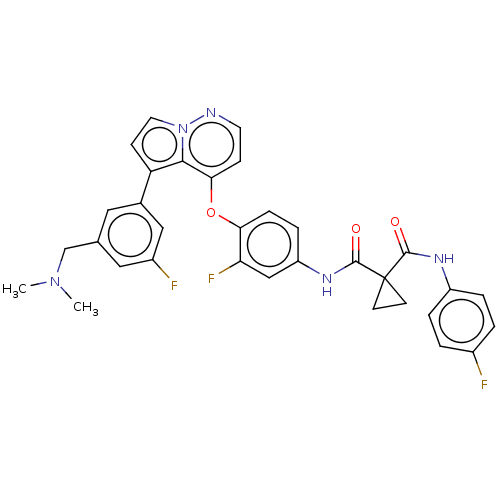
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
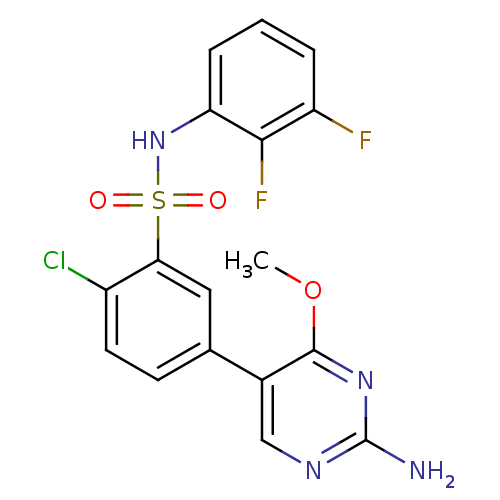
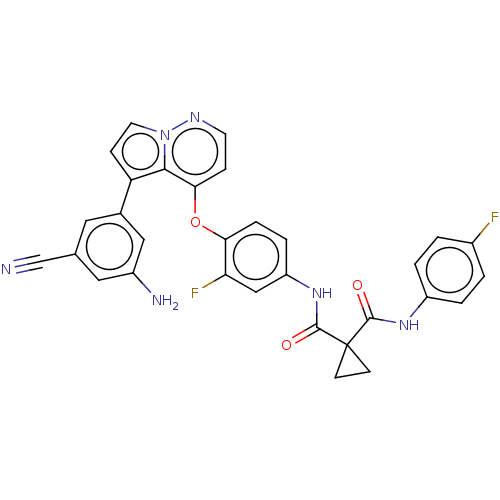
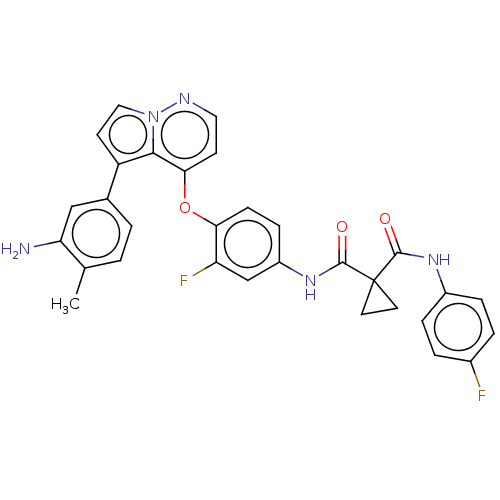
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human PI3Kalpha expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescen...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of human PI3Kbeta expressed in sf9 cells coexpressing p85alpha assessed as amount of ATP consumed by luciferase-luciferin chemiluminescenc...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Exelixis
Curated by ChEMBL
Exelixis
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Displacement of biotinylated geldanamycin from human His-tagged Hsp90 by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair
Affinity DataIC50: <40nMAssay Description:The inhibitory effect of the compounds of the present invention on the activity of c-Met was confirmed as follows.Specifically, 250 μM G4Y1 pept...More data for this Ligand-Target Pair

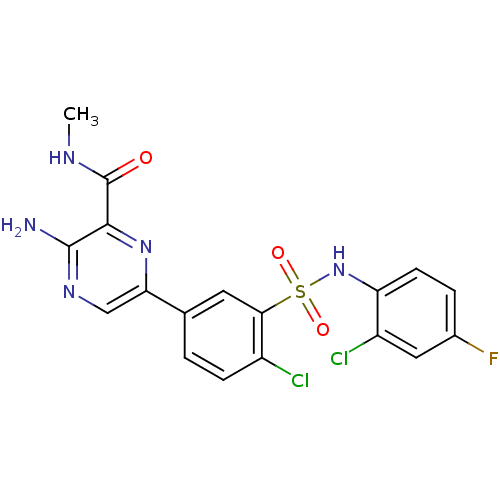

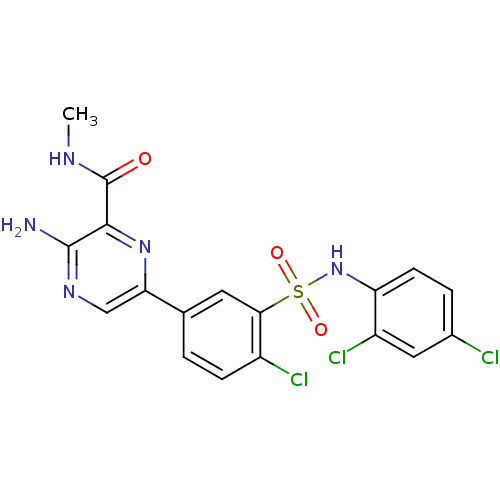




 3D Structure (crystal)
3D Structure (crystal)