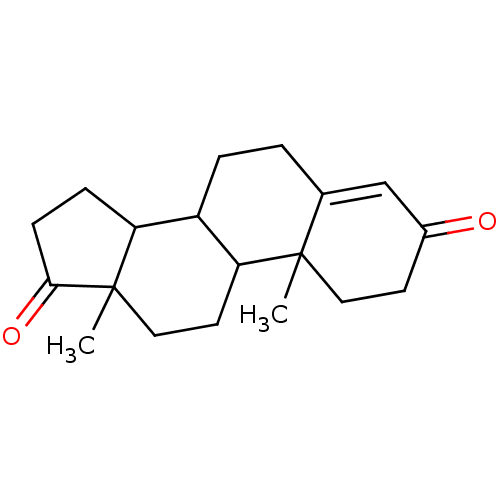
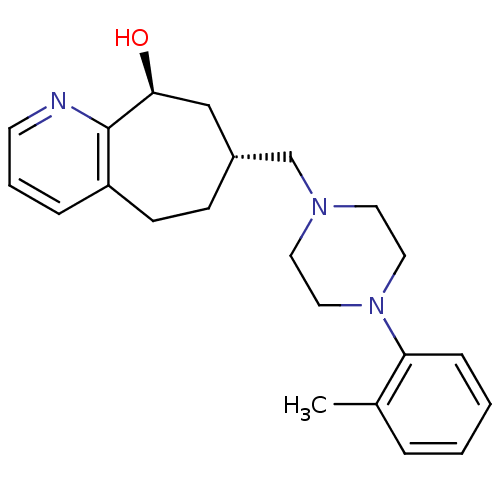
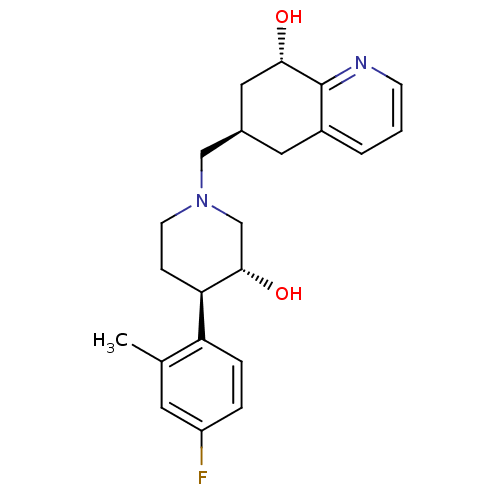
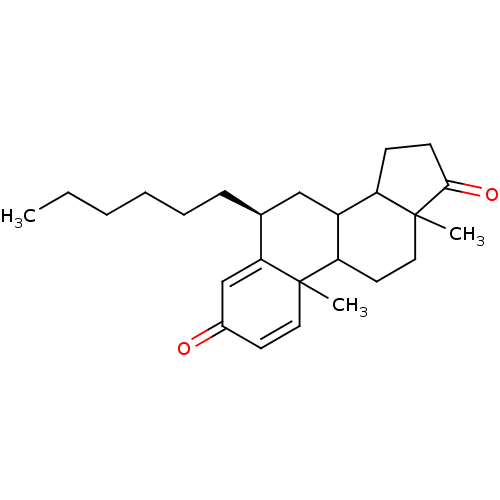
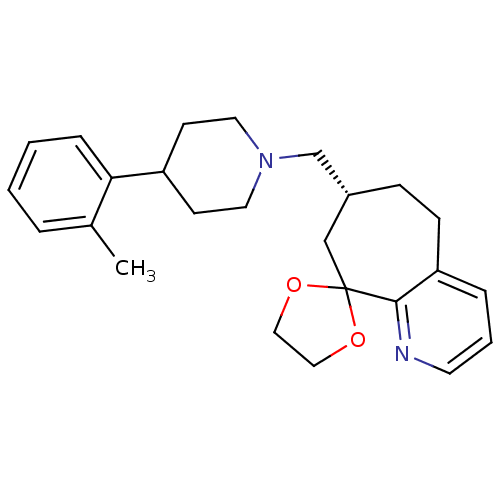
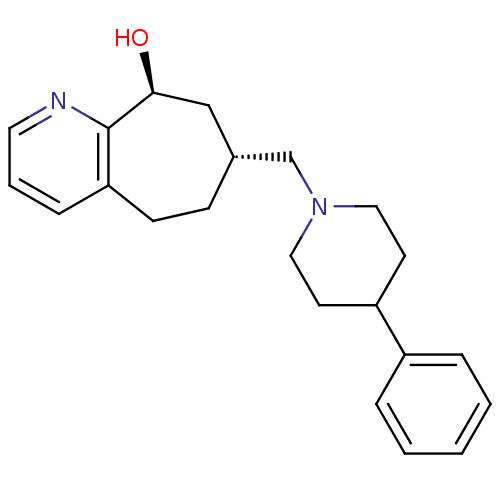
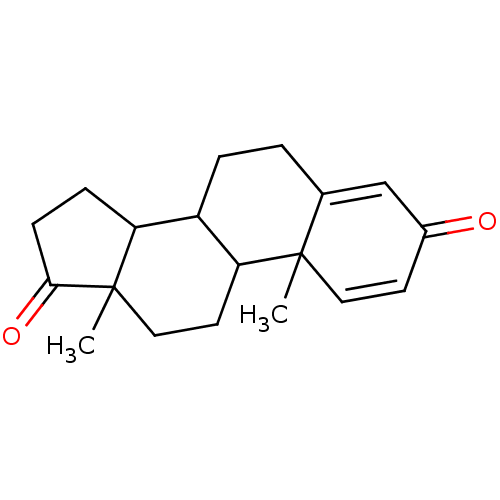
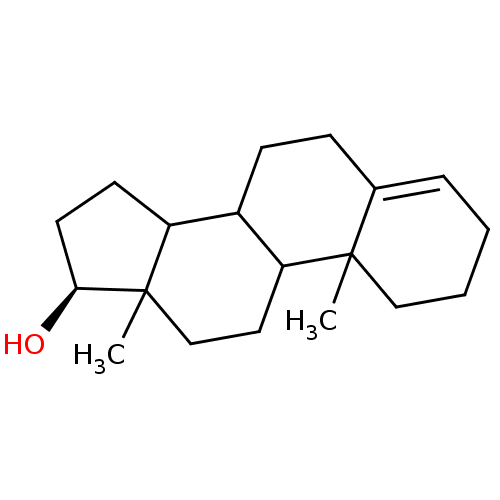
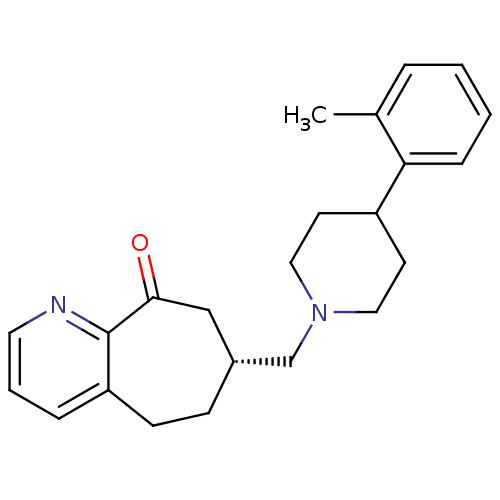
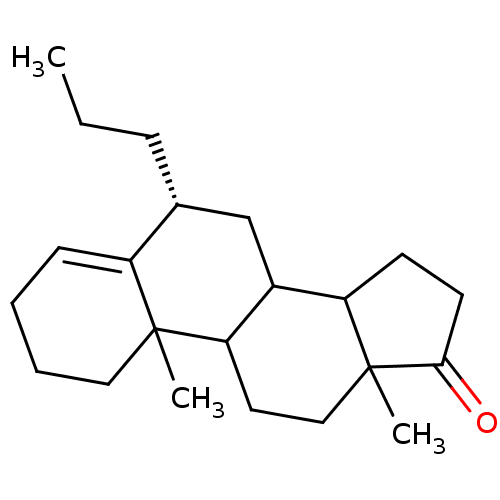
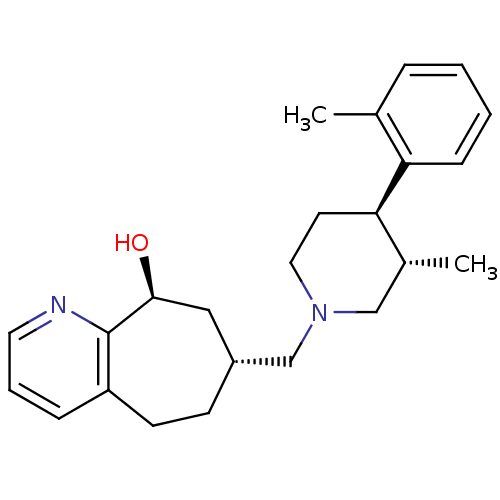
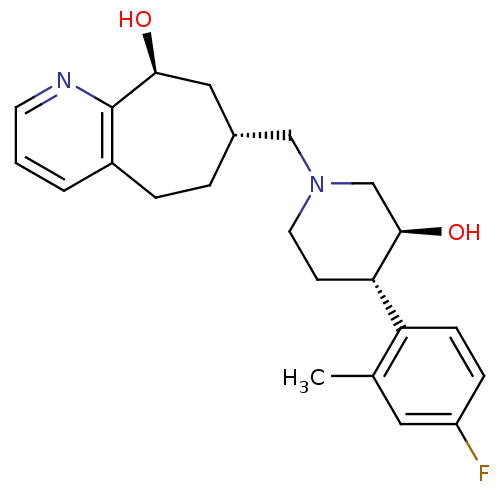
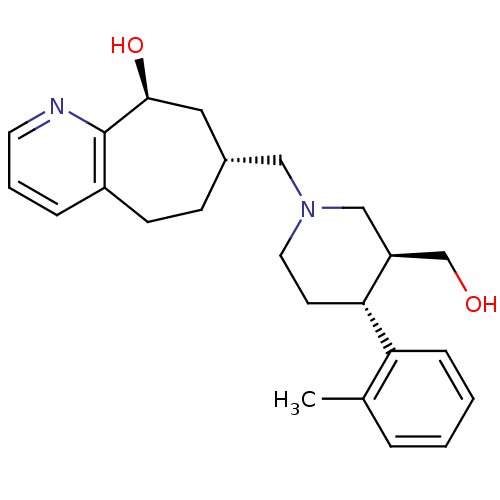
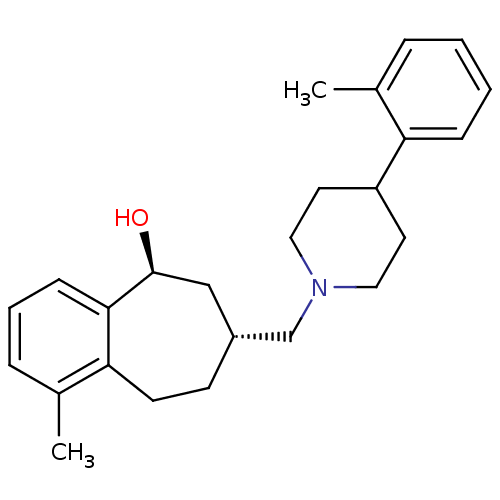
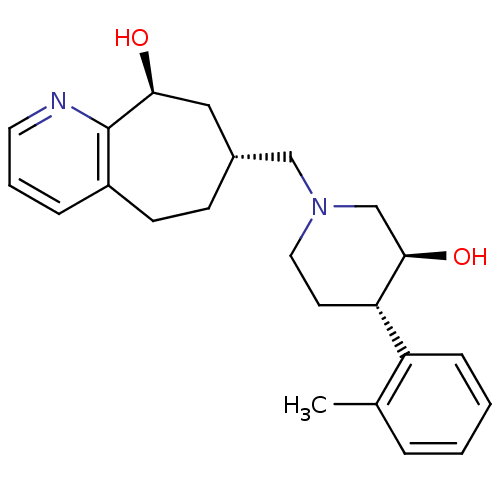
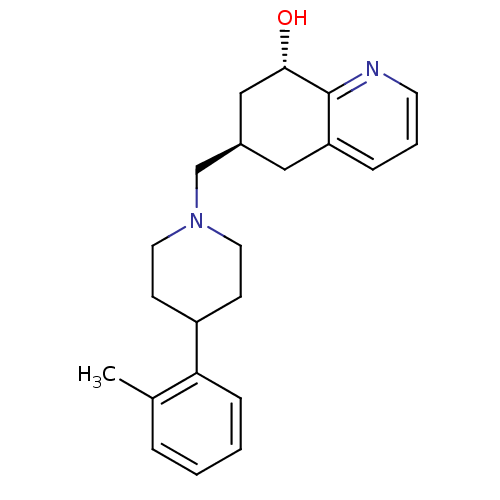
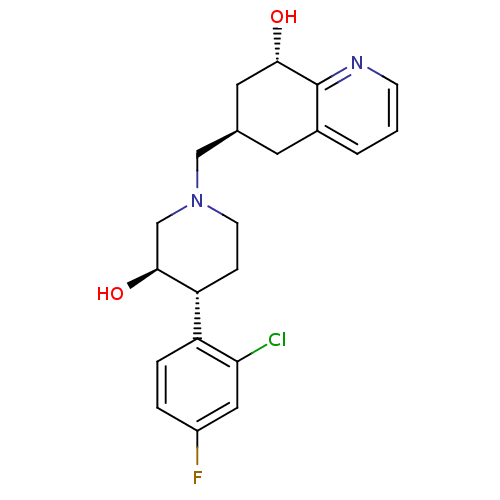
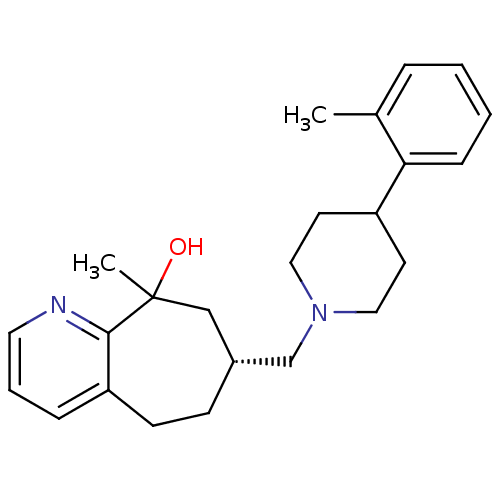
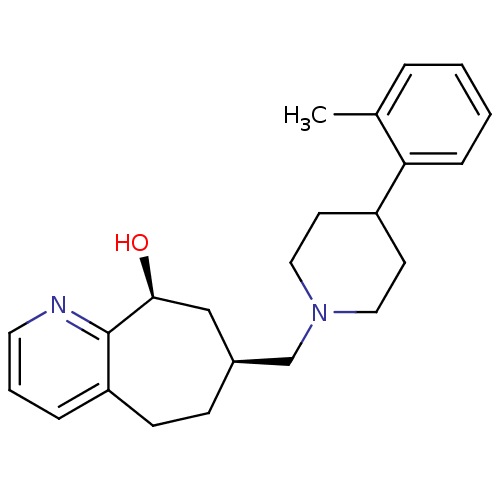
Affinity DataKi: 0.470nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
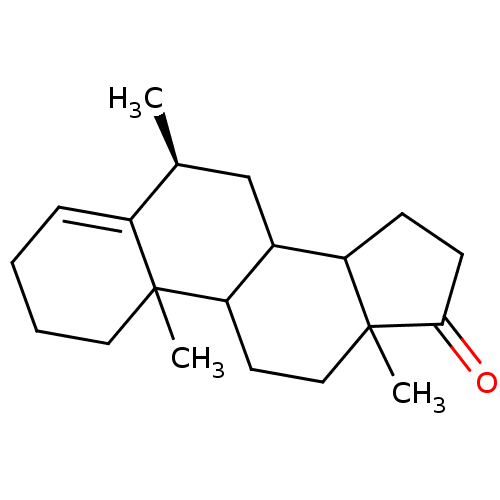
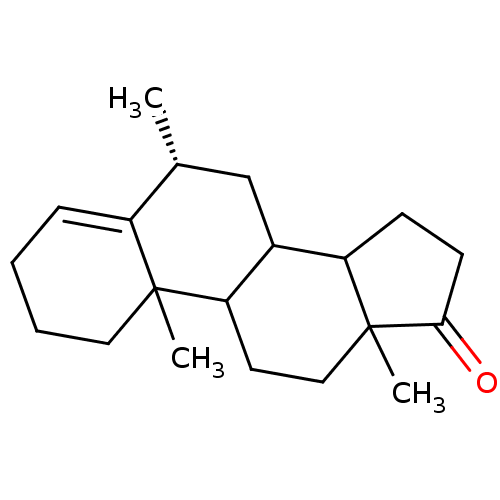
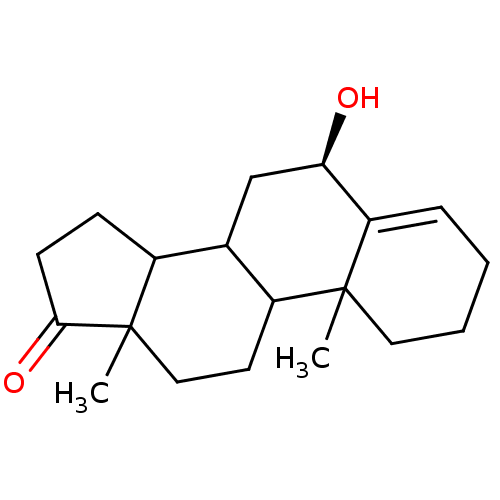
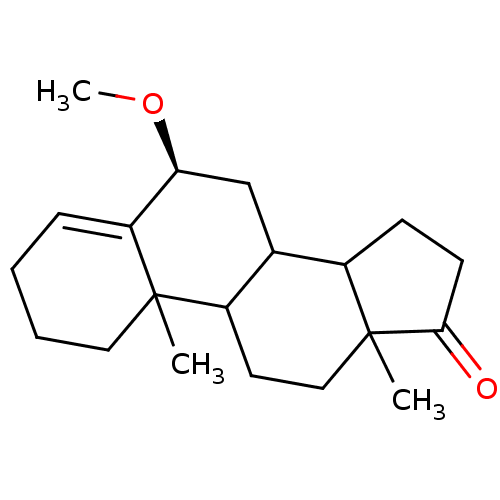
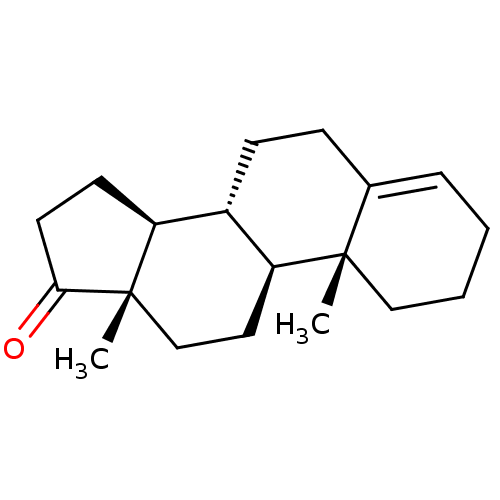
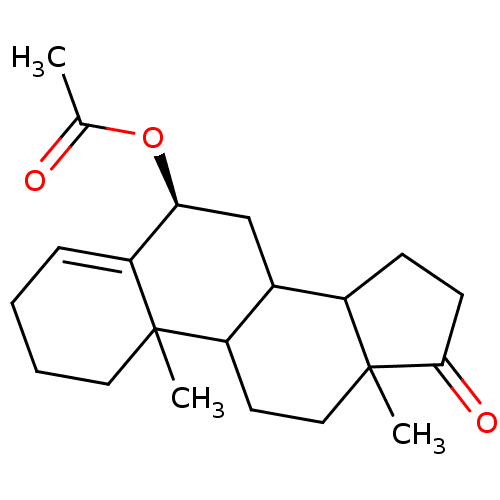
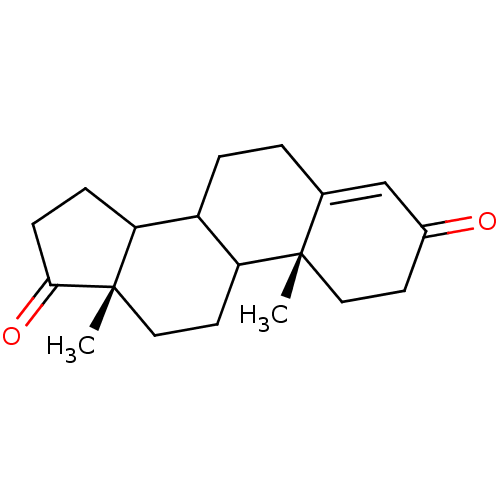
Affinity DataKi: 3.10nM ΔG°: -50.5kJ/mole IC50: 37nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nM ΔG°: -49.4kJ/mole IC50: 54nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5nM ΔG°: -49.3kJ/mole IC50: 54nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5.30nM ΔG°: -49.1kJ/mole IC50: 49nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -48.8kJ/mole IC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nM IC50: 60nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 7nM ΔG°: -48.4kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 7.80nM ΔG°: -48.1kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -48.1kJ/mole IC50: 76nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -47.3kJ/mole IC50: 140nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -47.0kJ/mole IC50: 120nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 12nM IC50: 130nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plotMore data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -46.6kJ/mole IC50: 110nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -46.6kJ/mole IC50: 180nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 16nM ΔG°: -46.3kJ/mole IC50: 200nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -46.0kJ/mole IC50: 160nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 19nM ΔG°: -45.8kJ/mole IC50: 250nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -45.7kJ/mole IC50: 250nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -45.7kJ/mole IC50: 300nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plotMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -45.6kJ/mole IC50: 190nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 22nM ΔG°: -45.5kJ/mole IC50: 270nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 24nM IC50: 260nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -45.1kJ/mole IC50: 280nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 30nM ΔG°: -44.7kJ/mole IC50: 230nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -43.7kJ/mole IC50: 320nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -43.7kJ/mole IC50: 520nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 43nM ΔG°: -43.7kJ/mole IC50: 530nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 45nM IC50: 360nMAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 51nM ΔG°: -43.3kJ/mole IC50: 440nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair

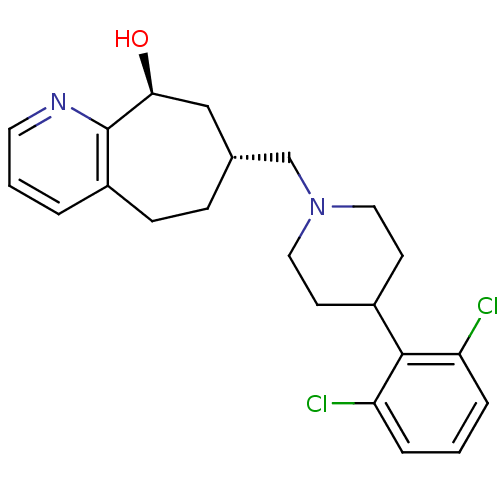
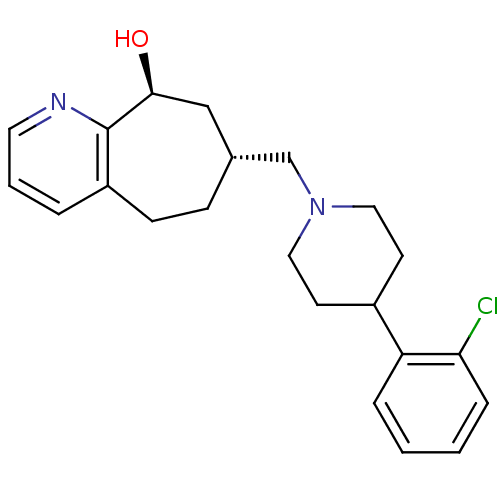














 3D Structure (crystal)
3D Structure (crystal)