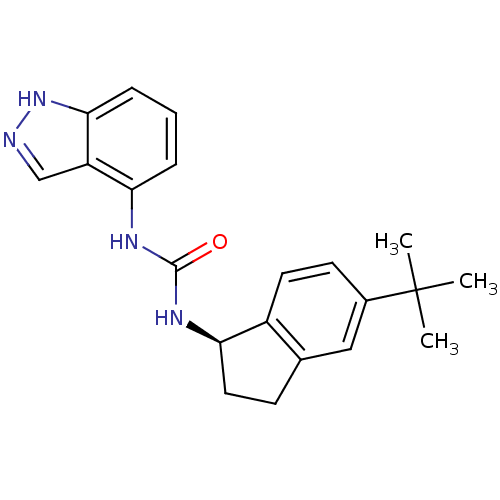
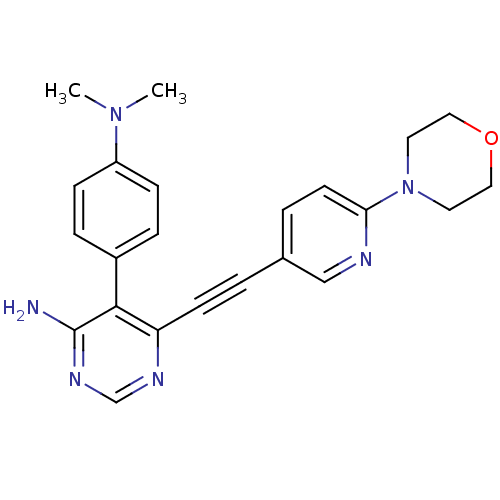
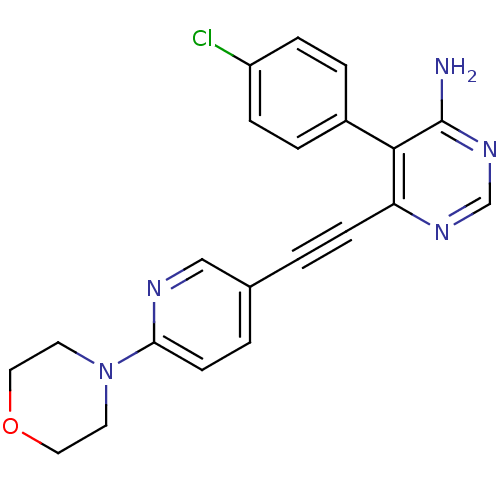
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 7nM ΔG°: -46.5kJ/mole EC50: 5nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
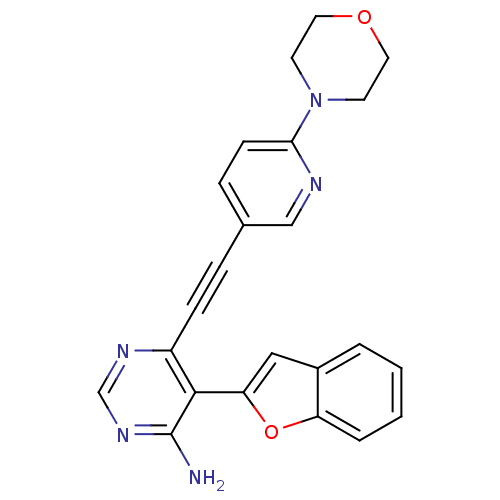
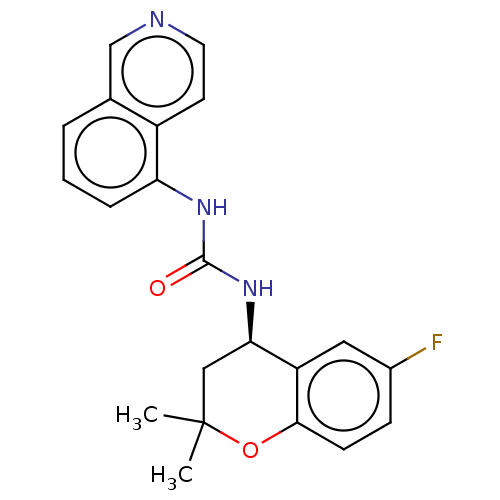
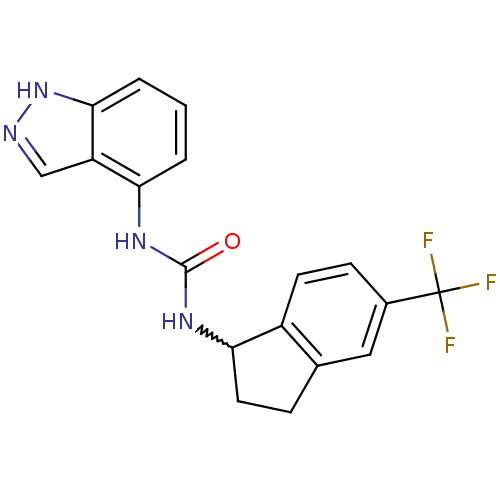
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 27nM ΔG°: -43.2kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
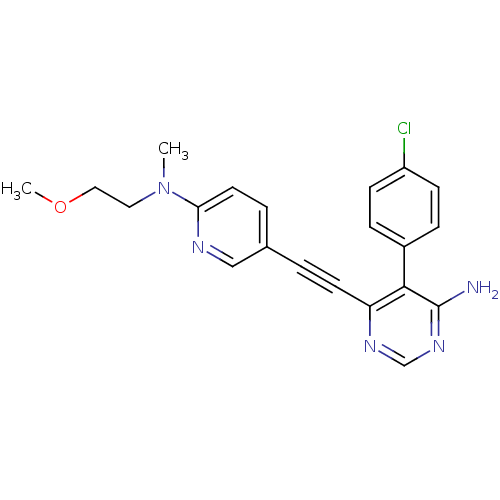
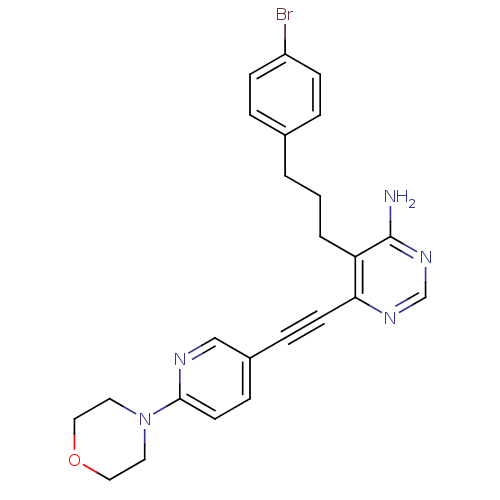
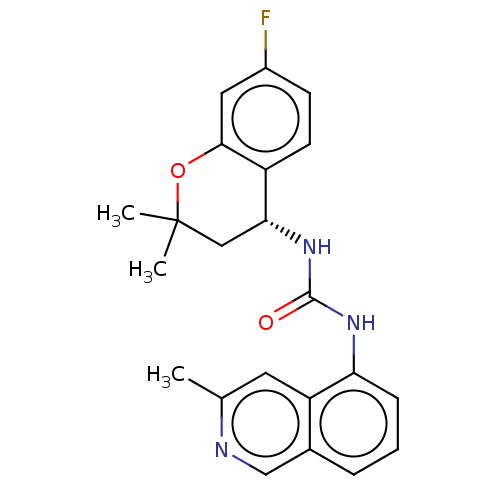
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 47nM ΔG°: -41.8kJ/mole EC50: 11nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
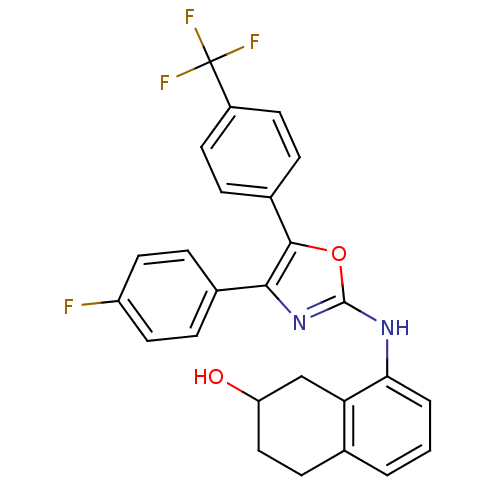
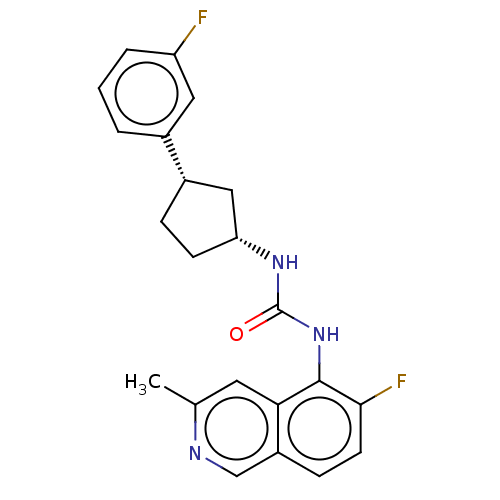
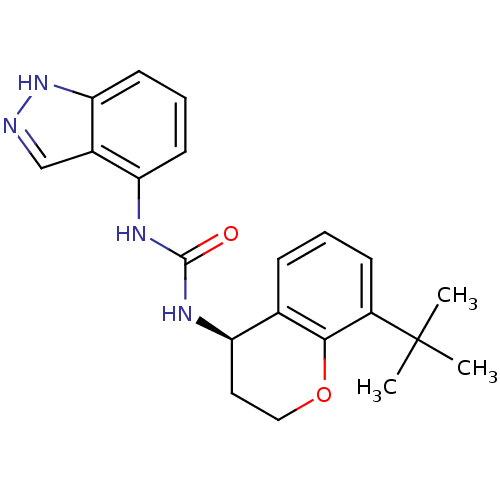
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 58nM ΔG°: -41.3kJ/mole EC50: 46nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 65nM ΔG°: -41.0kJ/mole EC50: 24nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 71nM ΔG°: -40.8kJ/mole EC50: 74nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 112nM ΔG°: -39.7kJ/mole EC50: 34nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 589nM ΔG°: -35.6kJ/mole EC50: 129nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 603nM ΔG°: -35.5kJ/mole EC50: 95nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.29E+3nM ΔG°: -33.6kJ/mole EC50: 282nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 1.59E+3nM ΔG°: -33.1kJ/mole EC50: 132nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: >6.31E+3nM ΔG°: >-29.7kJ/mole EC50: 1.48E+3nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2.00E+4nM ΔG°: -26.8kJ/mole EC50: 29nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 0.700nMpH: 5.5Assay Description:Blockade of pH 5.5-induced activation of TRPV1More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMpH: 7.5 T: 2°CAssay Description:ADK activity was monitored by a radiochemical assay, which measures the conversion of radioactive Ado ([U-14C] adenosine or [2-3H] adenosine) to AMP ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1nMAssay Description:The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of intact cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Tested for inhibitory activity against AK in an in vitro cell-free enzyme assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Evaluated in vitro for inhibitory activity against Adenosine kinase (AK)More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Evaluated in vitro for inhibitory activity against Adenosine kinase (AK)More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2.30nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Evaluated in vitro for inhibitory activity against Adenosine kinase (AK)More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Evaluated in vitro for inhibitory activity against Adenosine kinase (AK)More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMpH: 7.5 T: 2°CAssay Description:ADK activity was monitored by a radiochemical assay, which measures the conversion of radioactive Ado ([U-14C] adenosine or [2-3H] adenosine) to AMP ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Blockade of N-arachidonoyl-dopamine-induced activation of TRPV1More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of cytosolic adenosine kinaseMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Blockade of human TRPV1 receptor assessed as inhibition of capsaicin-induced calcium fluxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3nMAssay Description:The cDNA for human TRPV1 (hTRPV1) was isolated by reverse transcriptase-polymerase chain reaction (RT-PCR) from human small intestine poly A+ RNA sup...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.30nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of intact cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Evaluated in vitro for inhibitory activity against Adenosine kinase (AK)More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 3.5nMAssay Description:The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc...More data for this Ligand-Target Pair




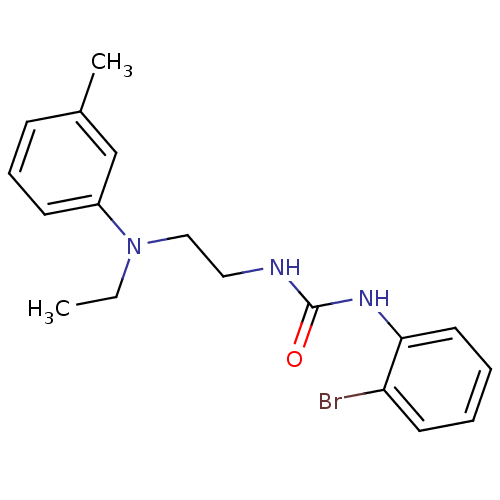
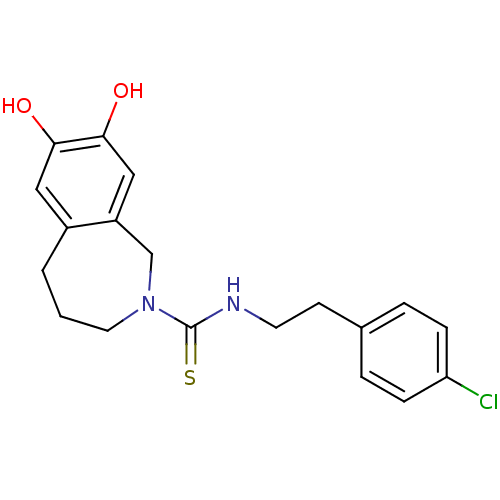
 3D Structure (crystal)
3D Structure (crystal)