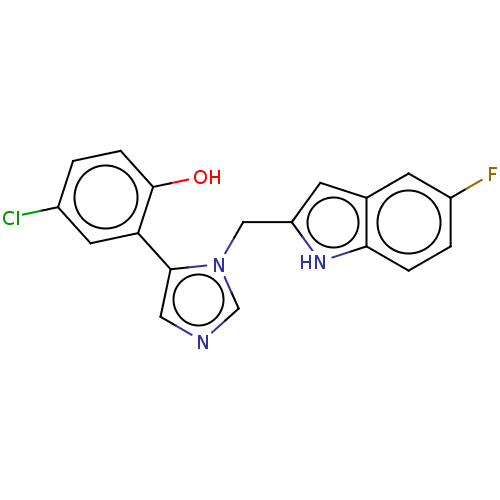
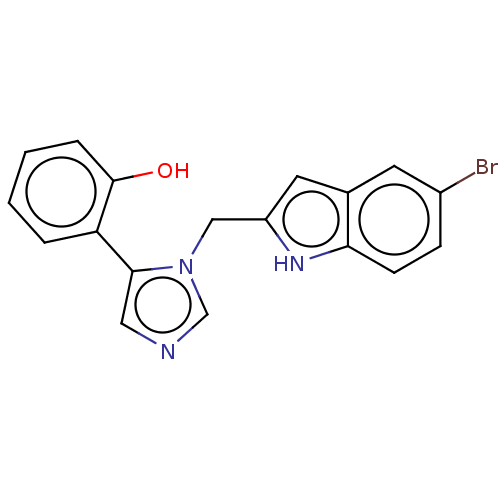
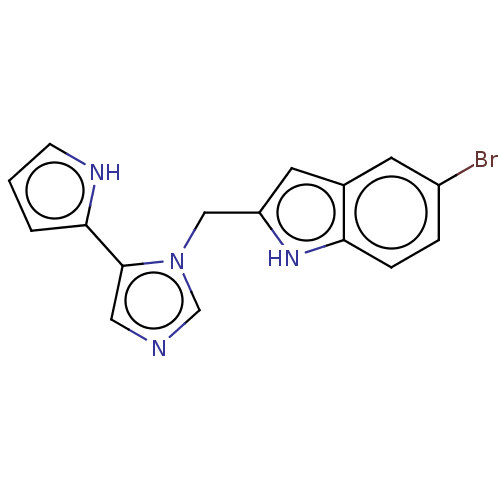
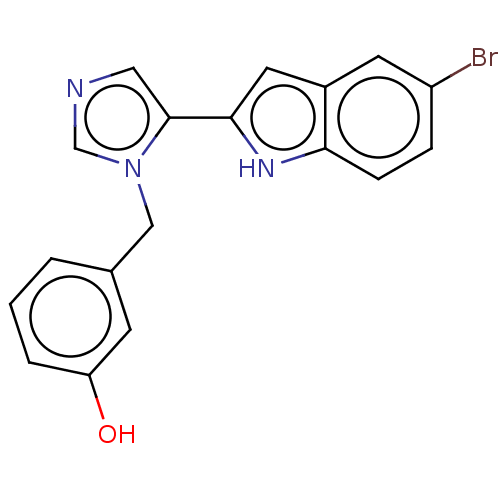
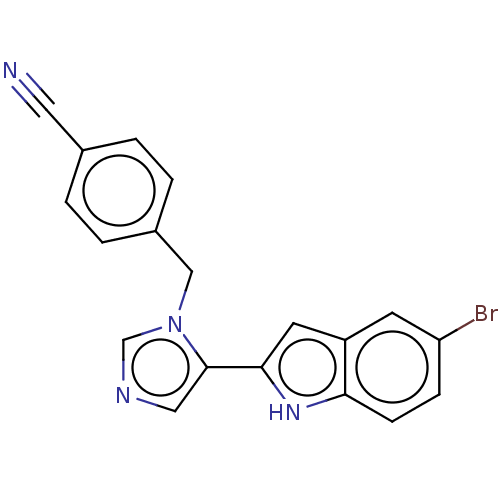
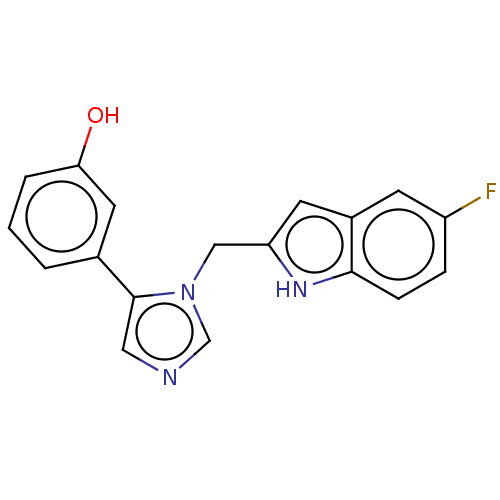
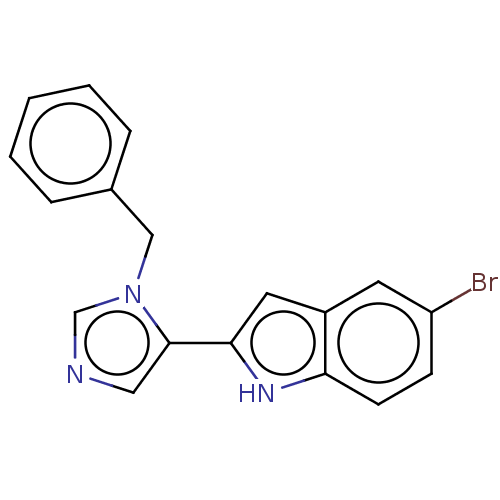
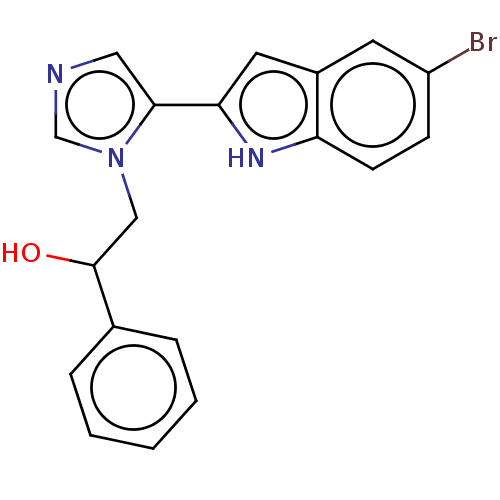
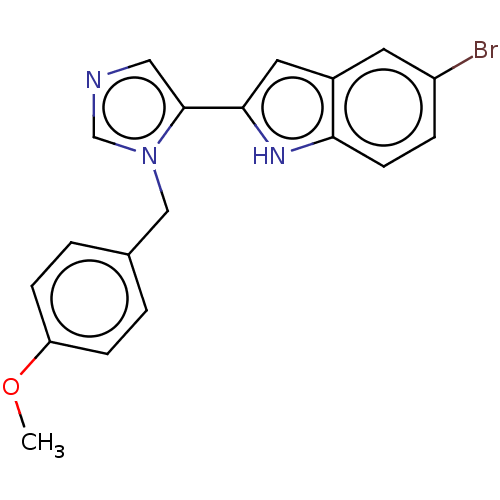
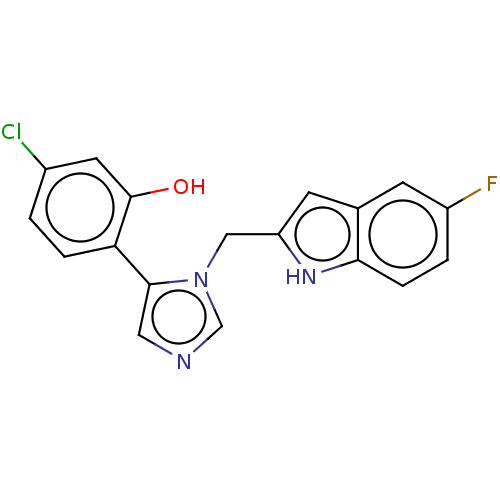
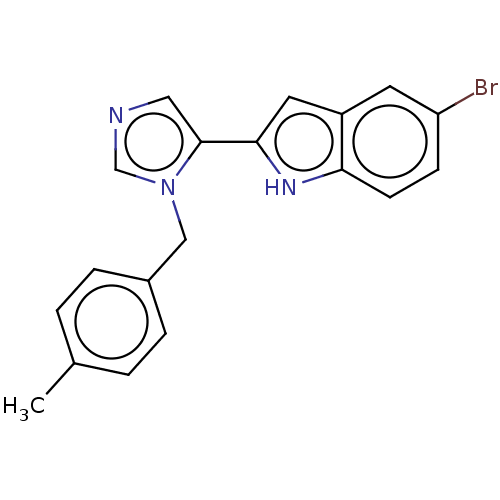
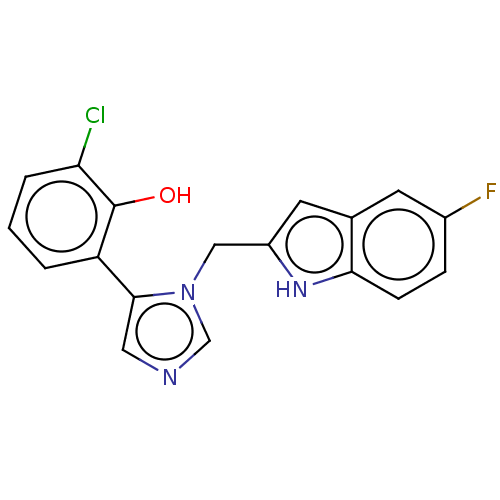
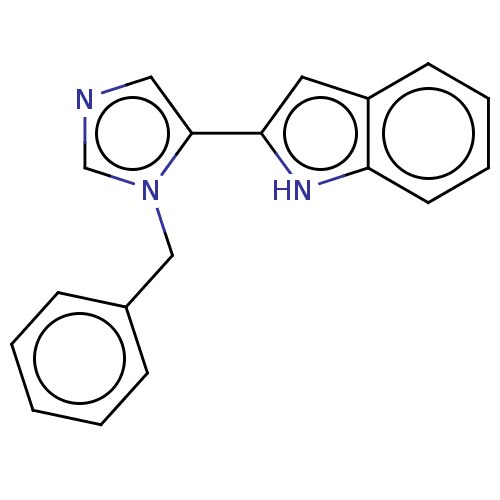
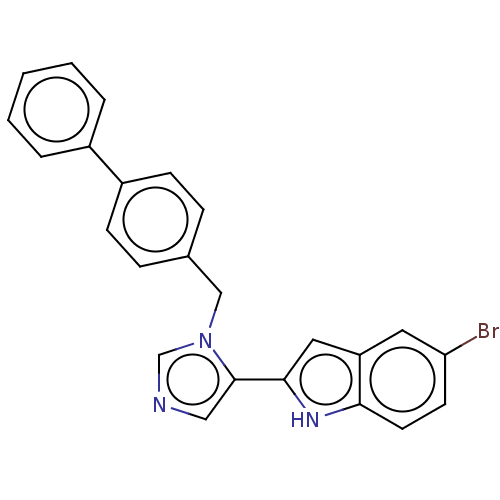
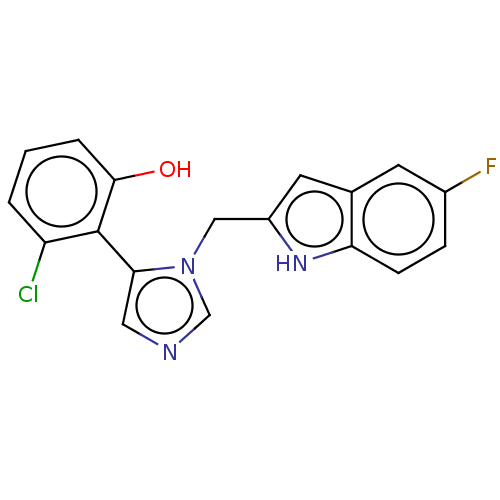
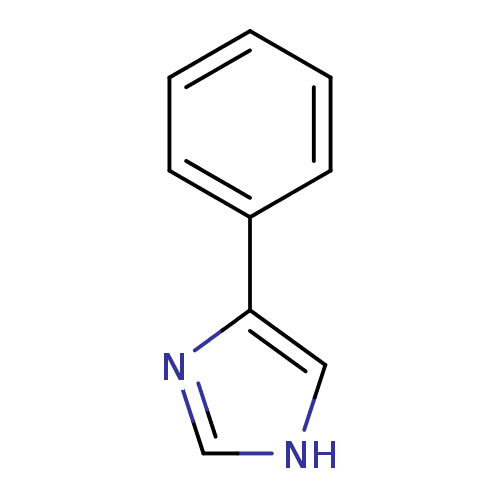
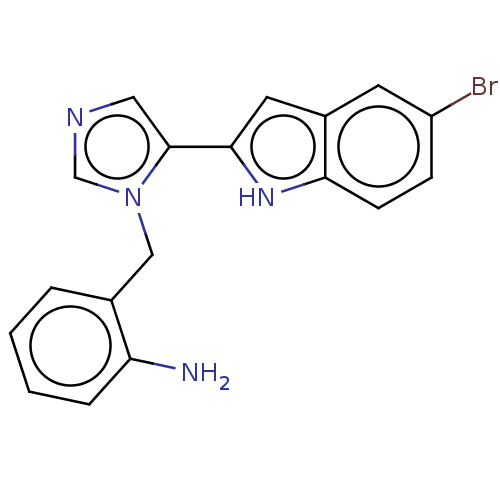
Affinity DataIC50: 34nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
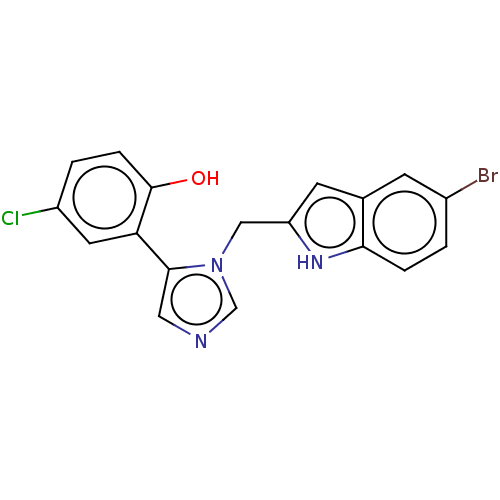
Affinity DataIC50: 38nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
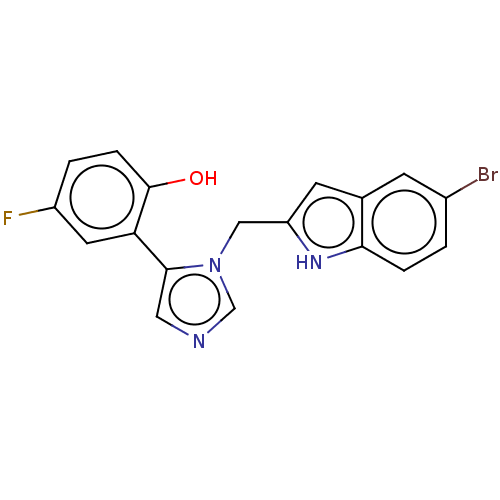
Affinity DataIC50: 100nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
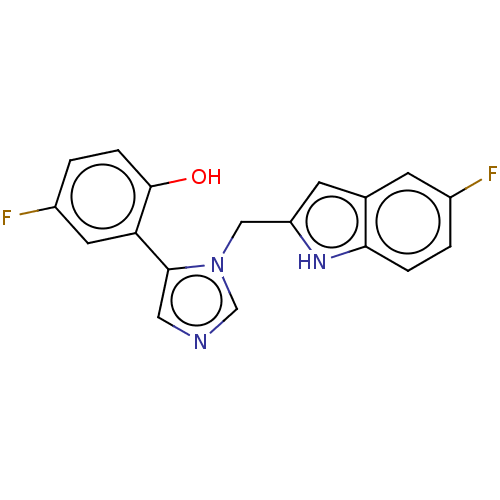
Affinity DataIC50: 113nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 113nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 180nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 180nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 309nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 322nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 322nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.81E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.44E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.44E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.64E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.64E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.16E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.27E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.27E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.42E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 3.48E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.25E+4nMAssay Description:A Standard reaction mixture (200 uL/well) containing 50 mM potassium phosphate buffer (pH 6.5), 20mM ascorbic acid (neutralized with NaOH), 200 ug/ml...More data for this Ligand-Target Pair
Affinity DataIC50: 4.48E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.48E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of human IDO1 expressed in Escherichia coli BL21 (DE3) using L-tryptophan as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:A standard reaction mixture (200uL/well) containing 50mM potassium phosphate buffer (pH 6.5), 20 mM ascorbic acid (neutralized with NaOH), 200 ug/mL ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.06E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 5.44E+4nMAssay Description:A Standard reaction mixture (200 uL/well) containing 50 mM potassium phosphate buffer (pH 6.5), 20mM ascorbic acid (neutralized with NaOH), 200 ug/ml...More data for this Ligand-Target Pair
Affinity DataIC50: 5.44E+4nMAssay Description:Inhibition of recombinant human 6His-tagged TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in kynurenine production using L-tryp...More data for this Ligand-Target Pair
Affinity DataIC50: 7.01E+4nMAssay Description:A Standard reaction mixture (200 uL/well) containing 50 mM potassium phosphate buffer (pH 6.5), 20mM ascorbic acid (neutralized with NaOH), 200 ug/ml...More data for this Ligand-Target Pair
Affinity DataIC50: 8.33E+4nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 1 hrMore data for this Ligand-Target Pair