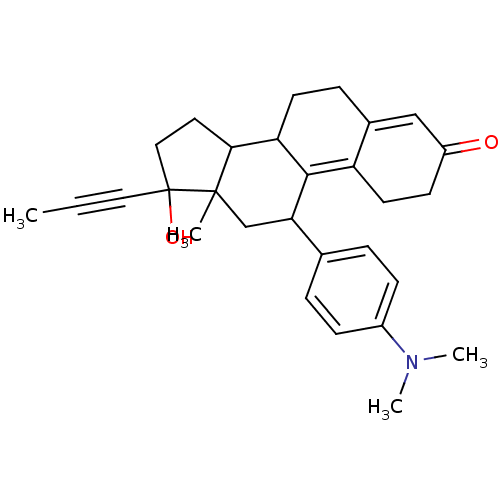
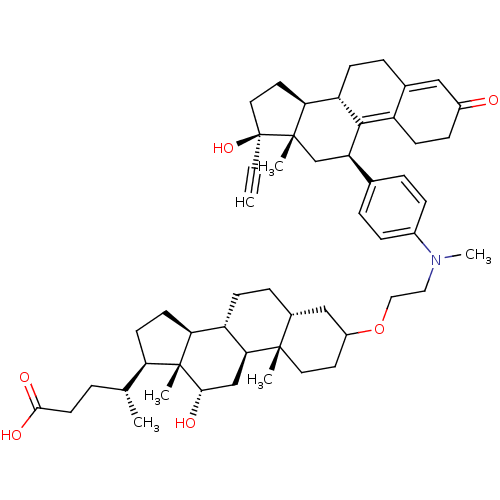
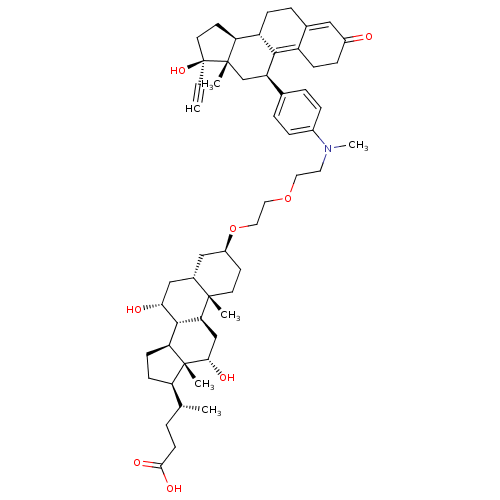
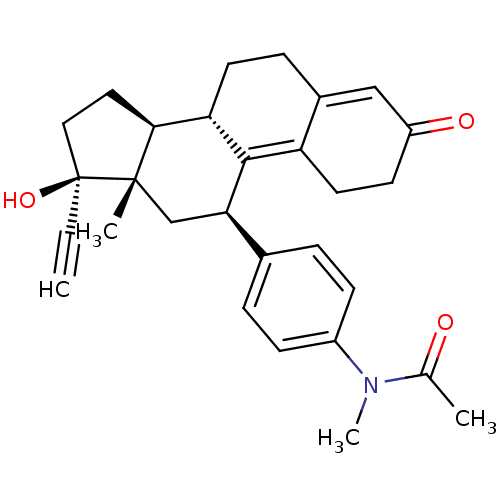
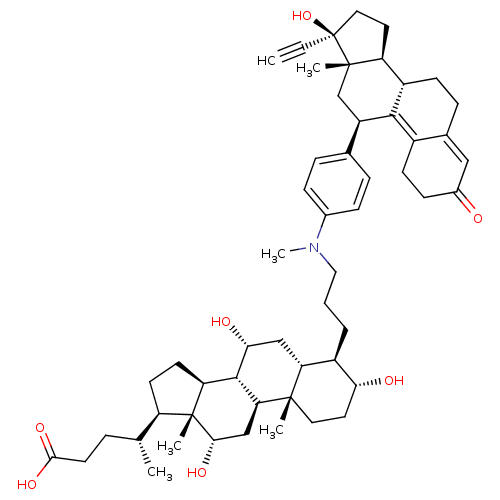
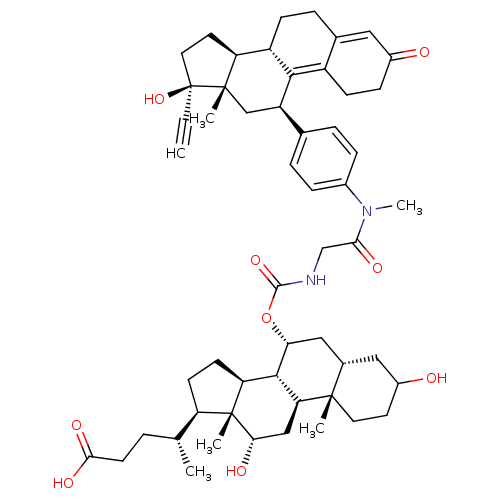
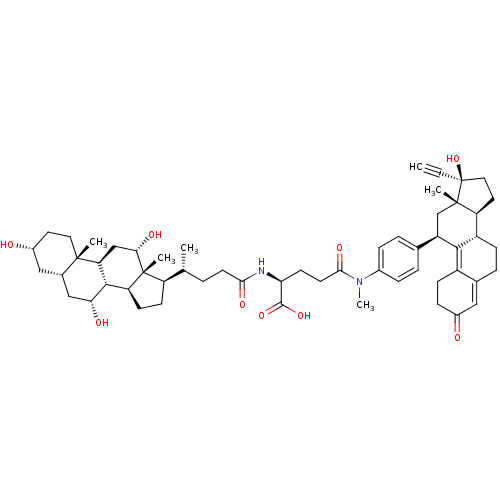
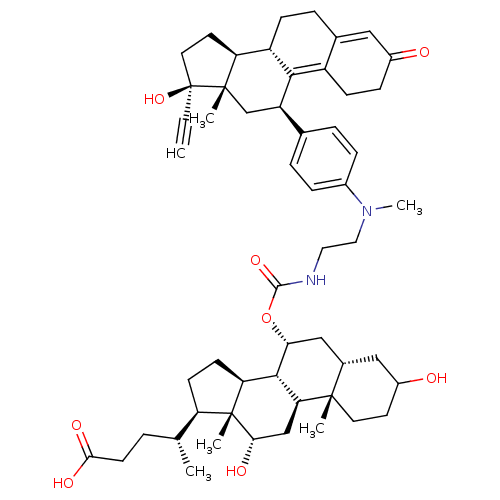
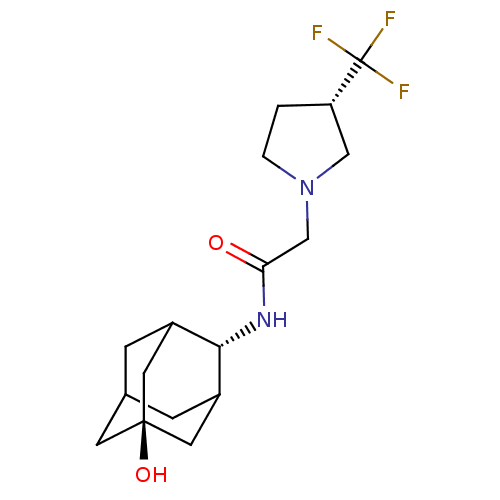
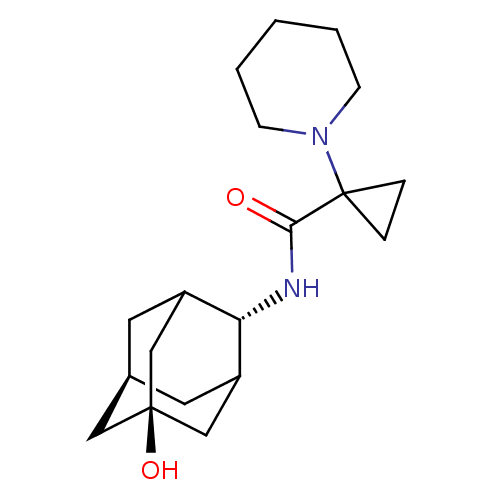
Affinity DataKi: 0.100nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.380nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
TargetMembrane-associated progesterone receptor component 1(RAT)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
Affinity DataKi: 0.640nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.650nMAssay Description:Inhibition of human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
TargetMembrane-associated progesterone receptor component 1(RAT)
Abbott Laboratories
Curated by PDSP Ki Database
Abbott Laboratories
Curated by PDSP Ki Database
Affinity DataKi: 0.960nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human glucocorticoid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of glucocorticoid receptor dependent alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisolMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisolMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisolMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPAMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPAMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPAMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)