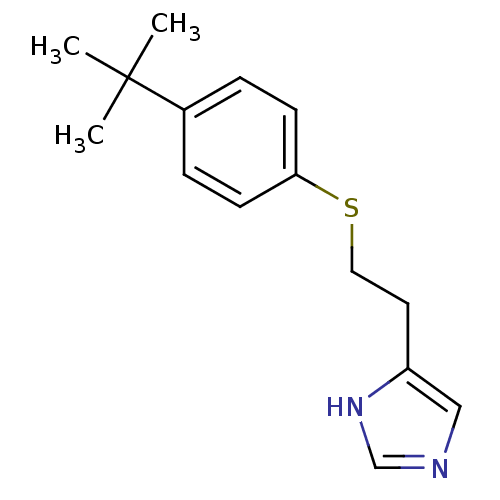
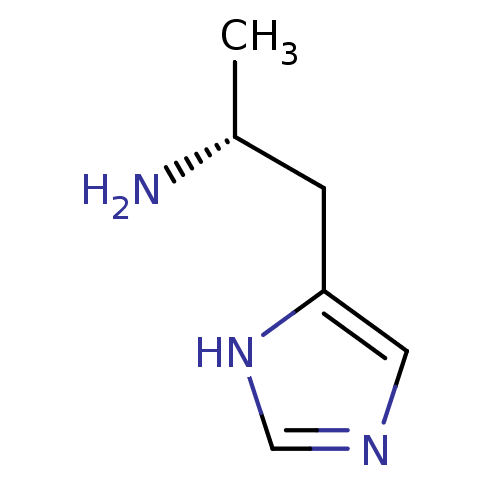
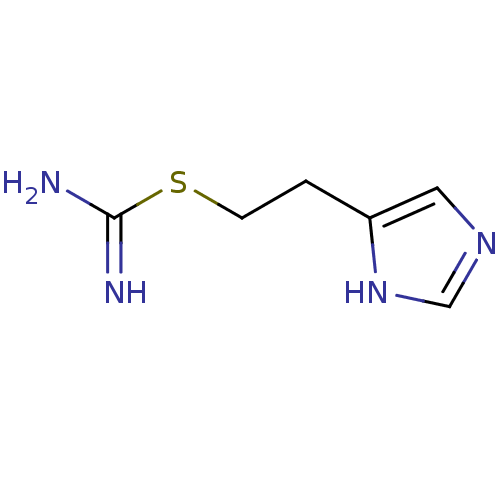
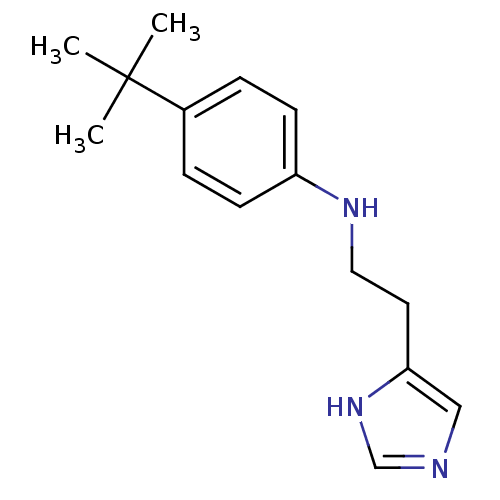
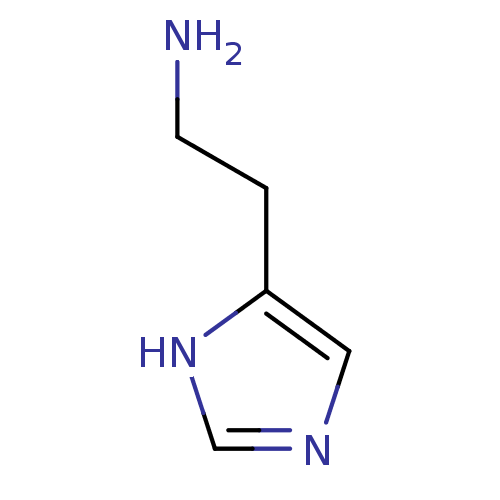
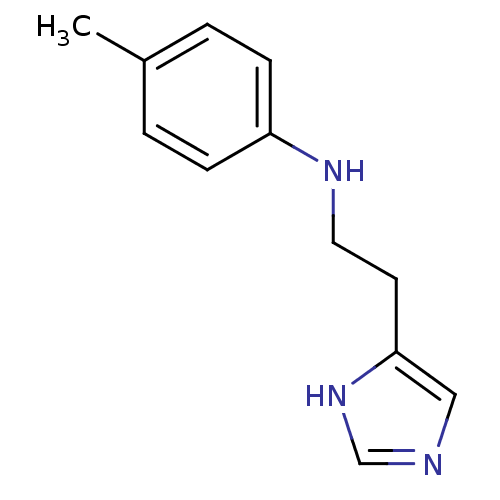
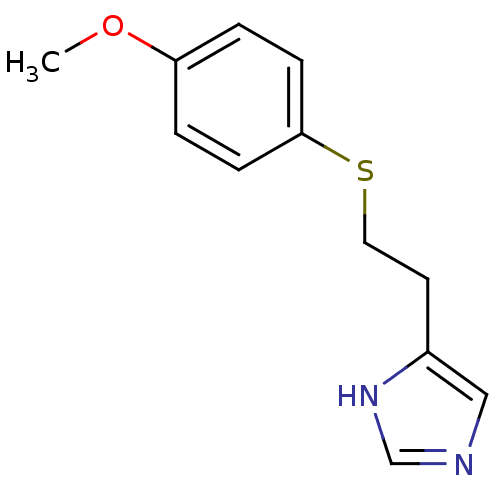
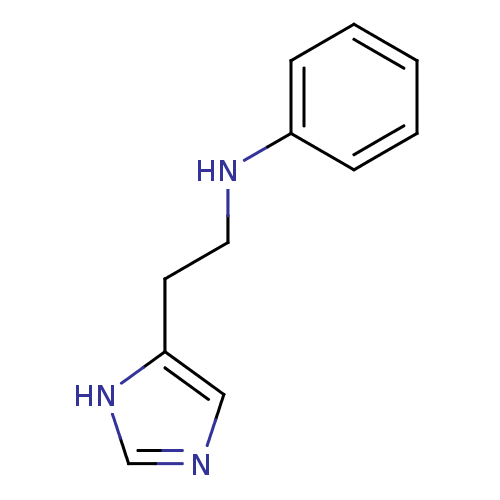
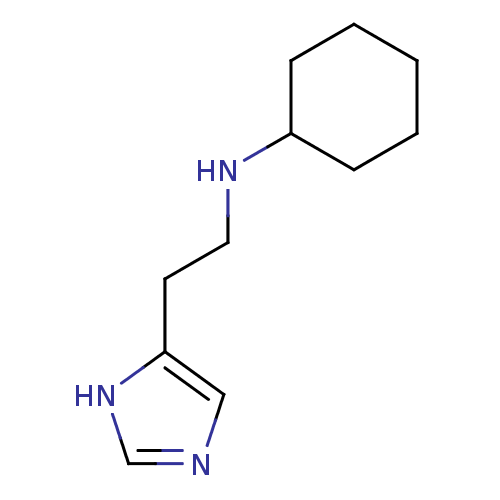
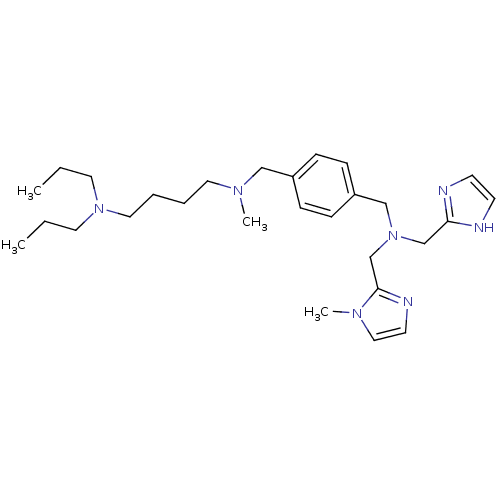
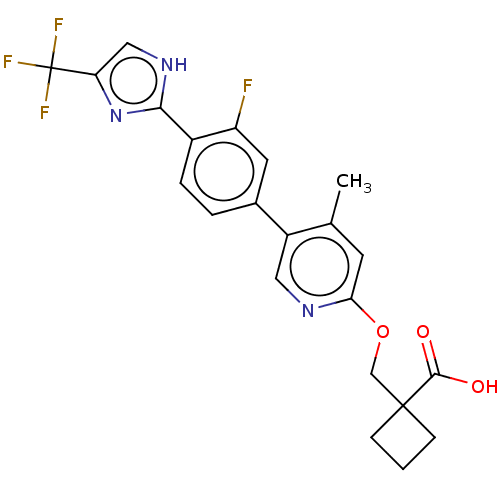
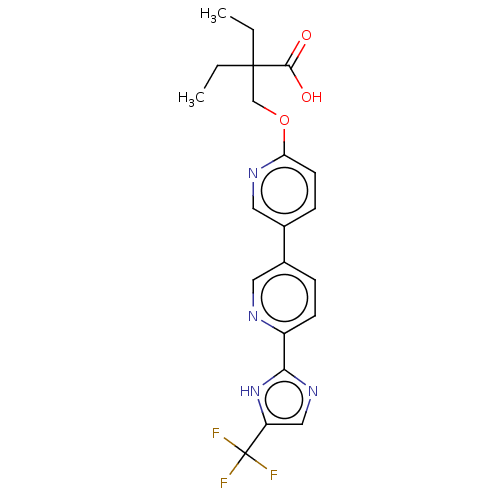
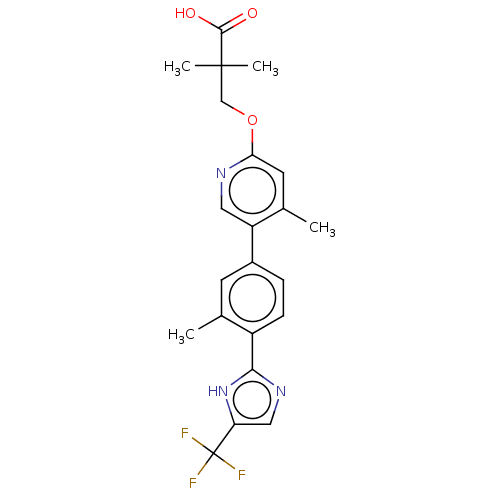
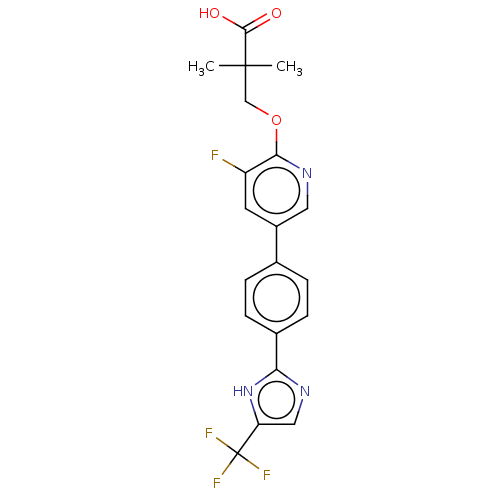
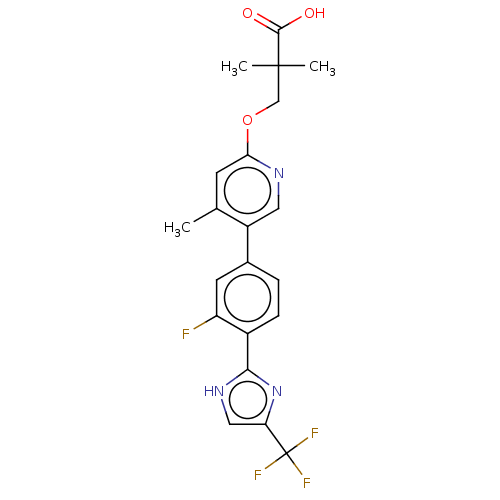
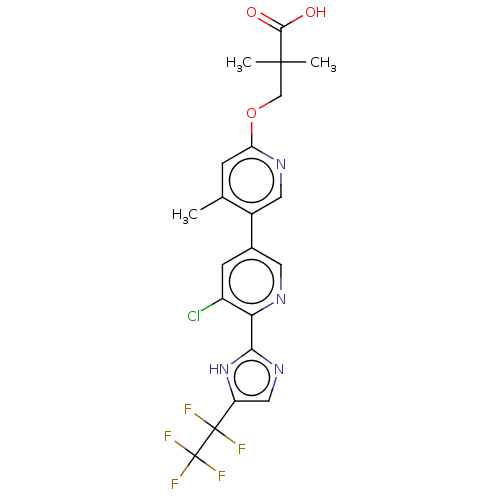
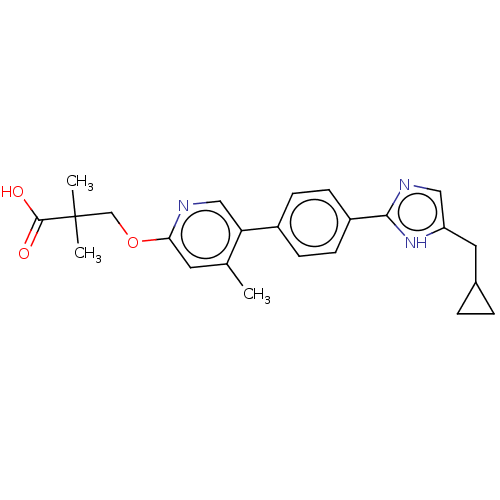
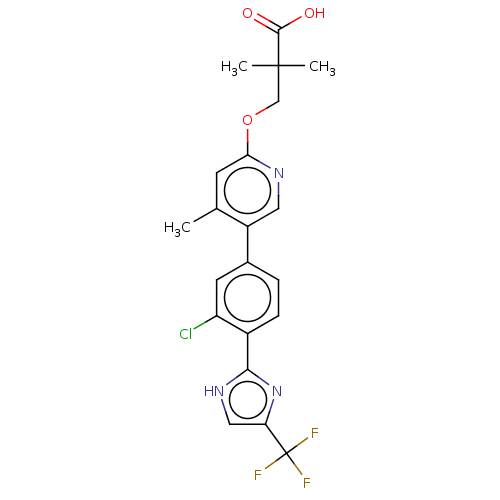
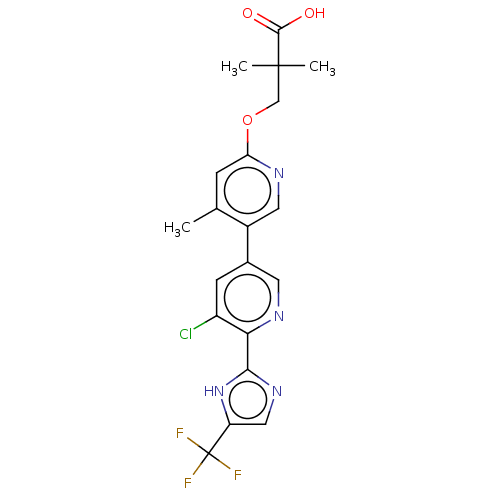
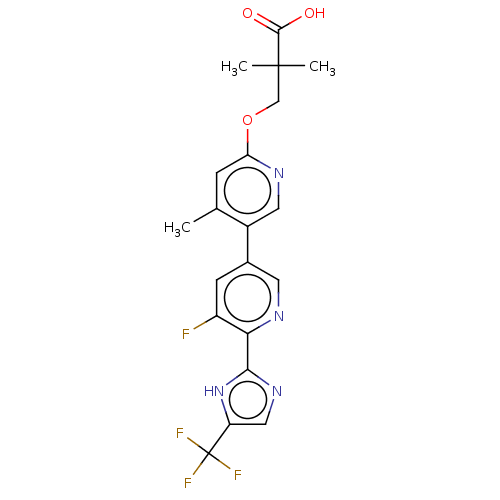
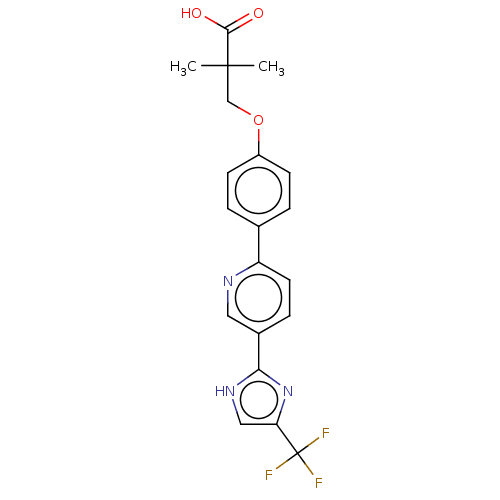
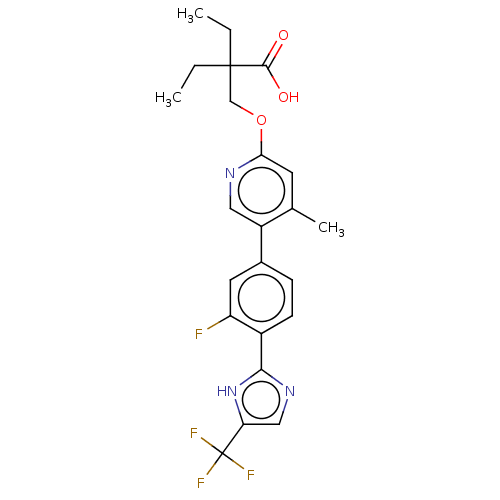
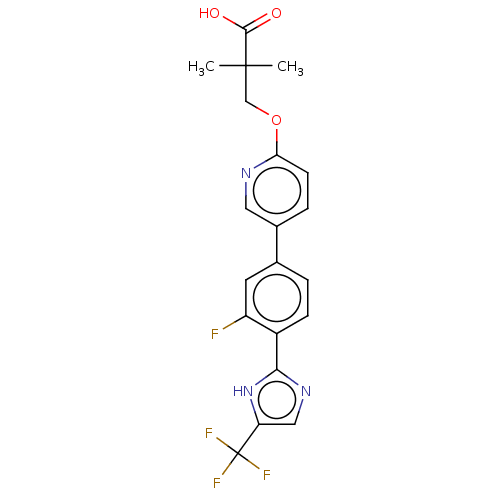
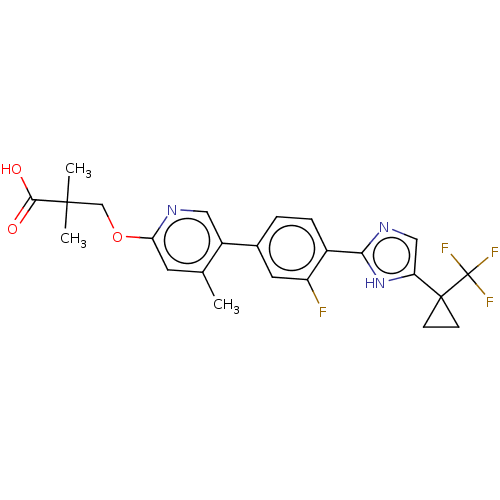
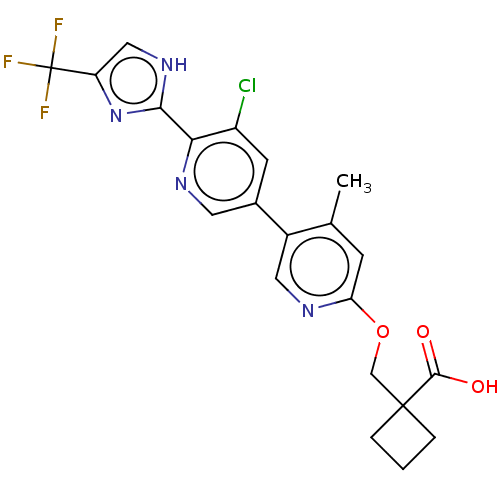
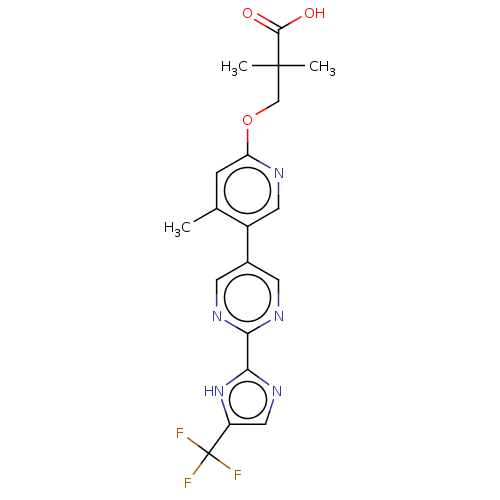
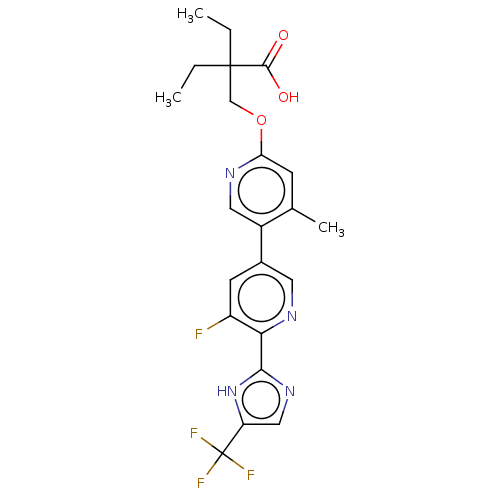
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.550nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant histamine H4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 4.10nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of beta2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of beta-1 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of muscarinic M3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Homo sapiens (Human))
National Institute Of Infectious Diseases
Curated by ChEMBL
National Institute Of Infectious Diseases
Curated by ChEMBL
Affinity DataIC50: 0.610nMAssay Description:Displacement of [125I]SDF-1alpha form CXCR4 expressed in CHO cells by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.870nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 7.4 T: 2°CAssay Description:As a buffer to be used in the enzymatic reaction of DGAT1, 100 mM Tris-HCl (pH 7.4), 200 mM Sucrose, 20 mM MgCl2, 0.125% Bovine Serum Albumin (BSA) w...More data for this Ligand-Target Pair