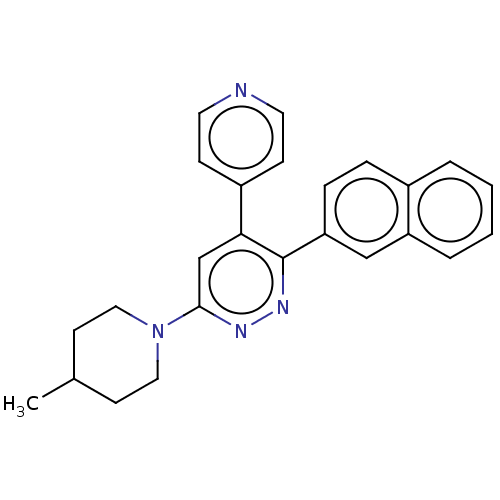
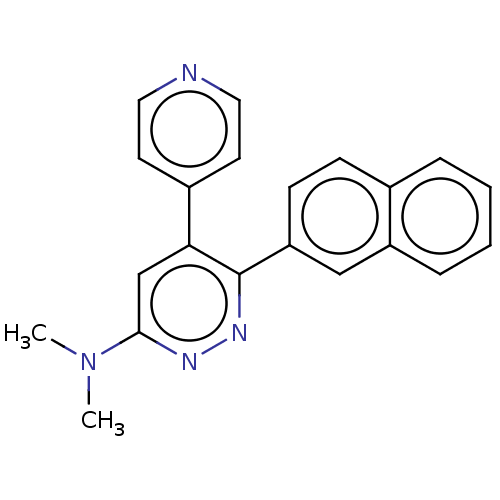
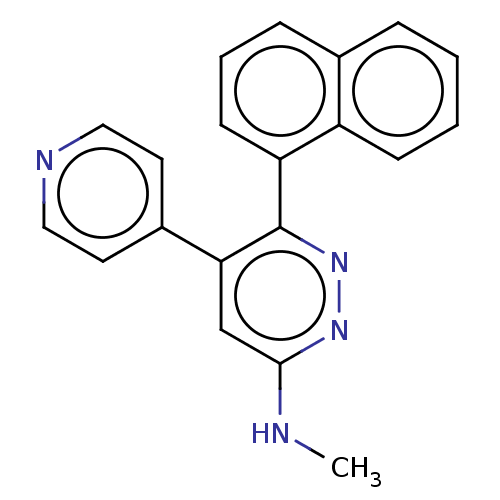
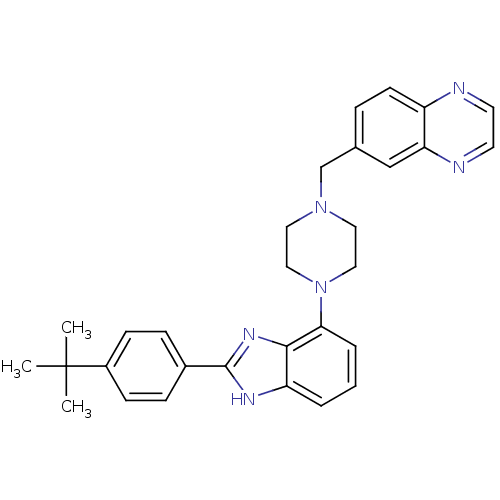
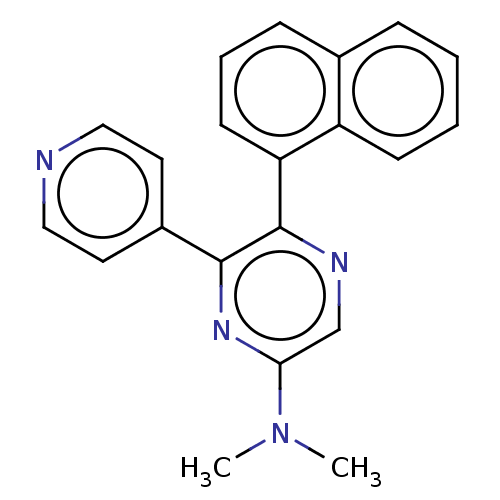
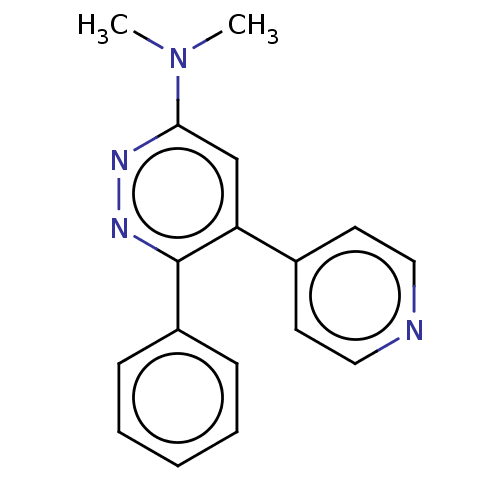
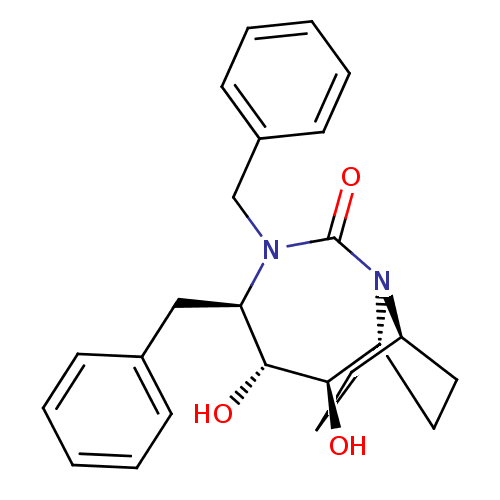
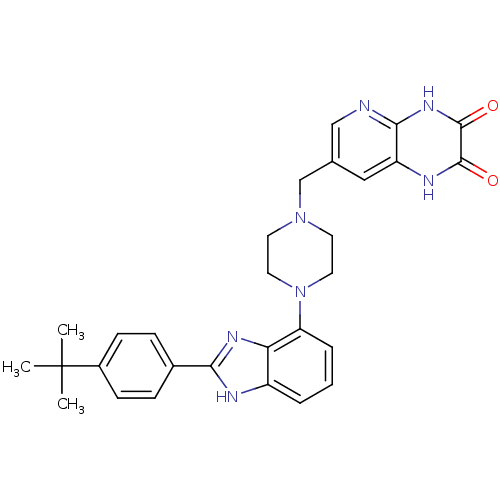
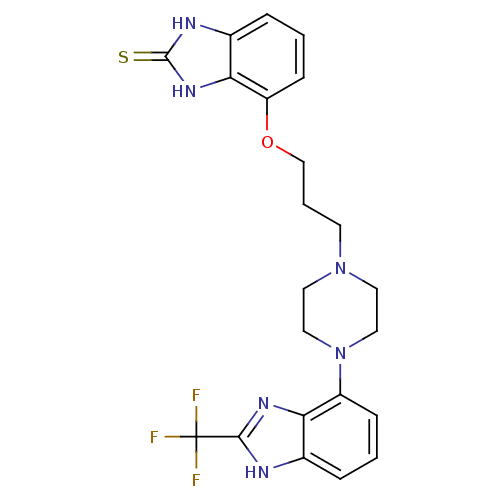
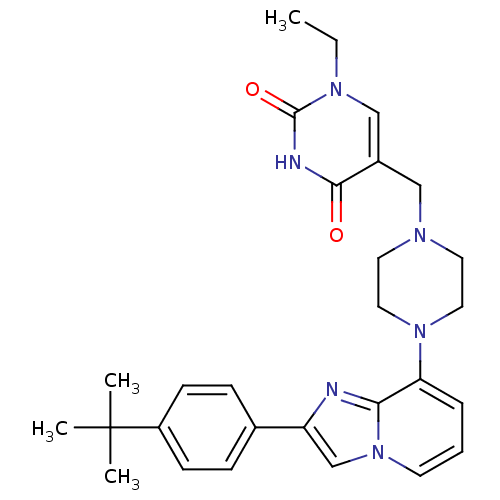
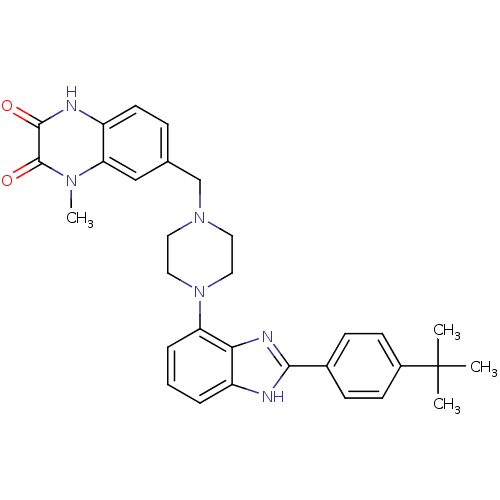
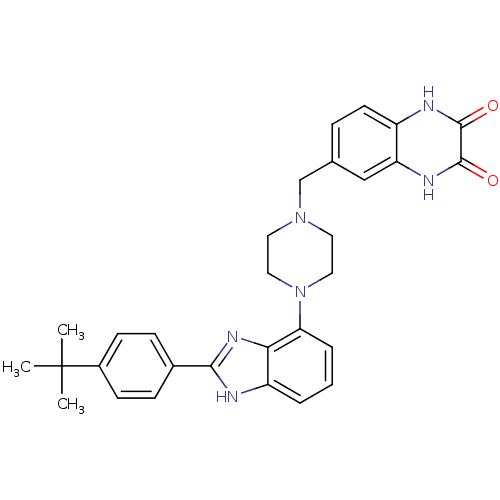
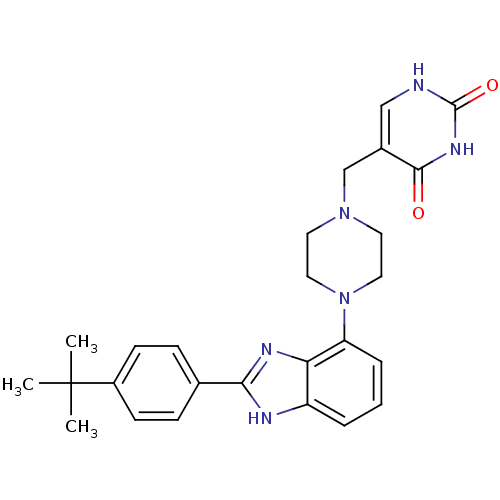
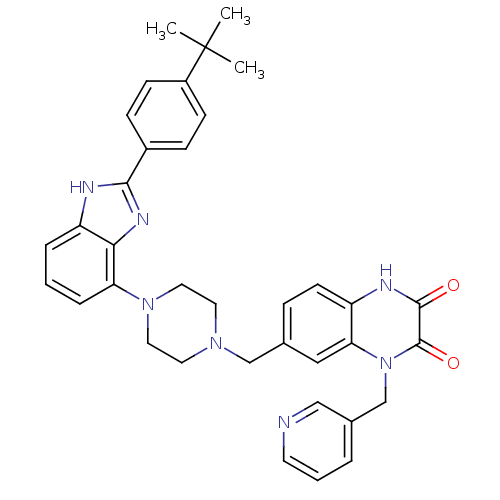
Affinity DataKi: 0.550nMAssay Description:Binding affinity to human 5HT1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibitory activity against HIV-1 Y181C reverse transcriptase.More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Binding affinity to human 5HT1B receptorMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 77nMAssay Description:Inhibition of Clostridium botulinum BoNT/A LC assessed as cleavage of SNAP-25 (141 to 206) after 30 mins by LC-MS analysisMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 86nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 91nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 98nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 100nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 100nMAssay Description:Inhibitory activity against HIV-1 Y188L reverse transcriptase.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 101nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 101nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 110nMAssay Description:Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190AMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 114nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 127nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 180nMAssay Description:Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236LMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 184nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 186nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Binding affinity to 5HT2A receptor (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 276nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11(Homo sapiens (Human))
Northwestern University
Curated by ChEMBL
Northwestern University
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Inhibitory activity against HIV-1 Y188L reverse transcriptase.More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibition of human neurokinin NK2 receptorMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 343nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 620nMAssay Description:Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103NMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
The Trustees of Columbia University In The City of New York
US Patent
The Trustees of Columbia University In The City of New York
US Patent
Affinity DataKi: 657nMAssay Description:The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 750nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
Affinity DataKi: 850nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.35E+3nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 6.30E+3nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.10E+4nMAssay Description:Compound was tested for the inhibition of HIV-1 protease by assaying the cleavage of a fluorescent peptide using HPLCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [125I]-(D-Trp6)-GnRH from human GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Displacement of [125I]D-Trp6-GnRH from human recombinant GNRH receptor by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human recombinant GnRH receptor expressed in HEK293 cells assessed as reduction in (D-Trp6)-GnRH-stimulated IP production by w...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human recombinant GnRH receptor assessed as reduction in (D-Trp6)LHRH-induced myo-(1,2)-[3H]inositol productionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Displacement of [125I]D-Trp6-GnRH from human recombinant GNRH receptor by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Antagonist activity at human recombinant GNRH receptor assessed as inhibition of D-Trp6-GNRH-induced IP accumulation after 1 hr by rapid filtration a...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptorMore data for this Ligand-Target Pair

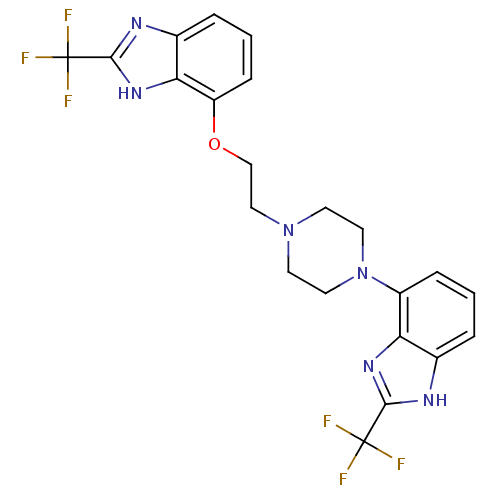
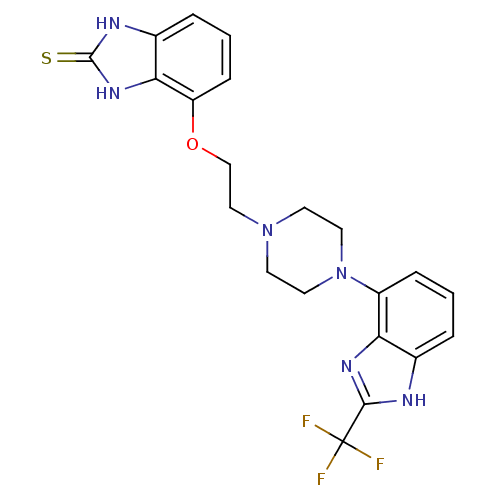
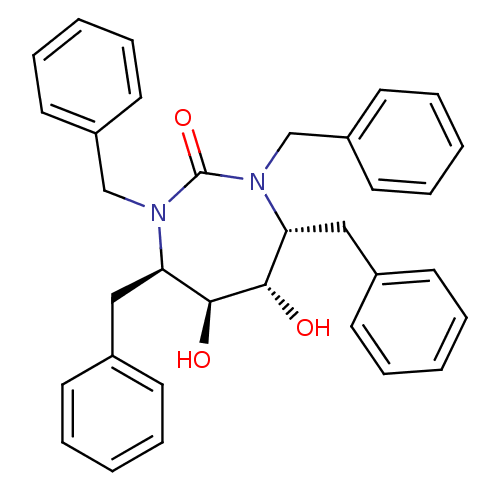

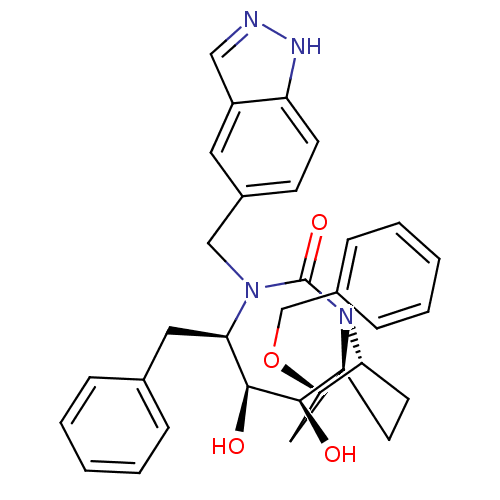


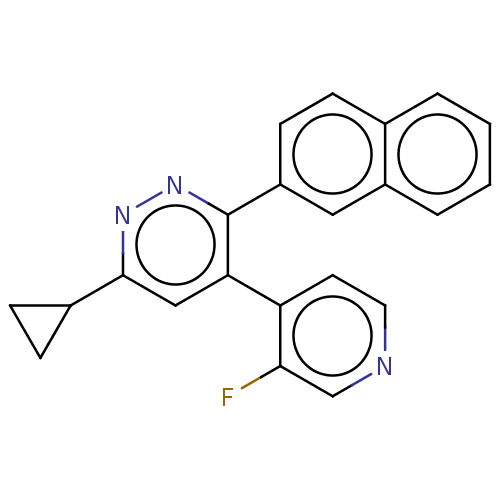
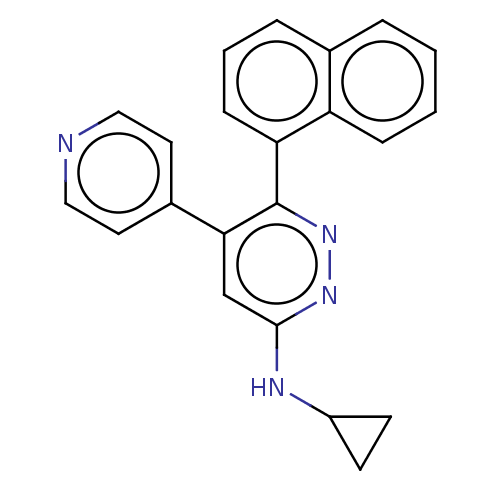
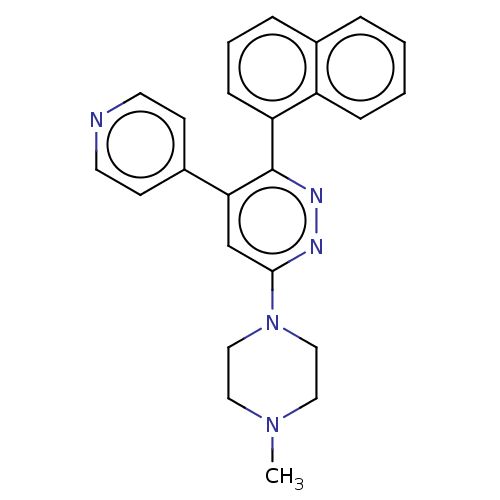
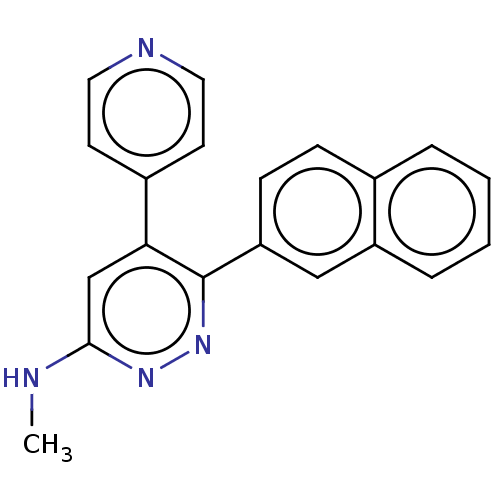

 3D Structure (crystal)
3D Structure (crystal)