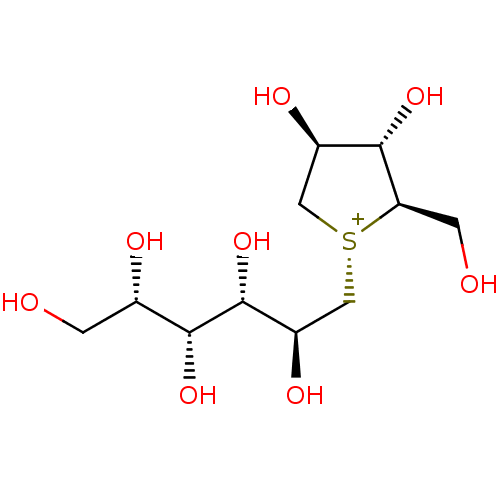
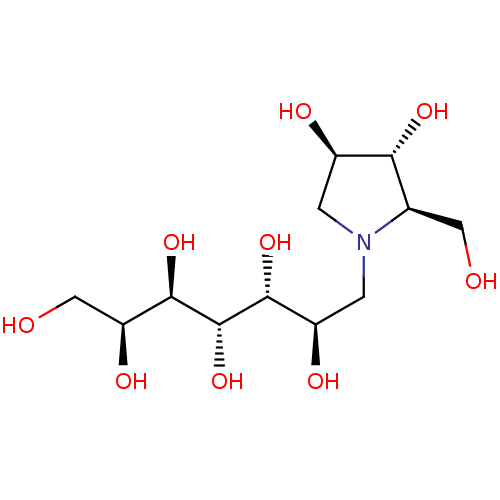
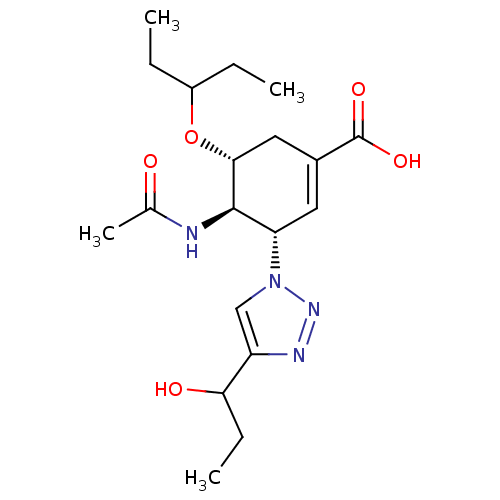
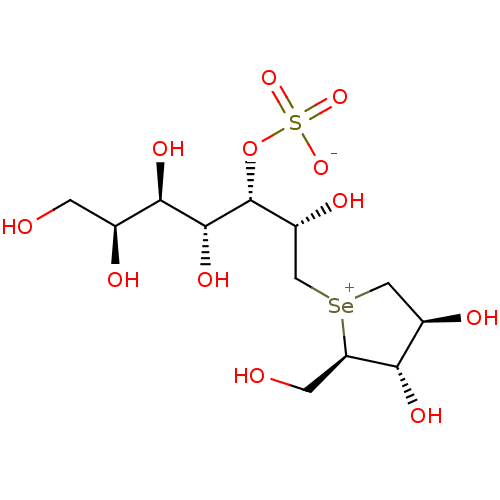
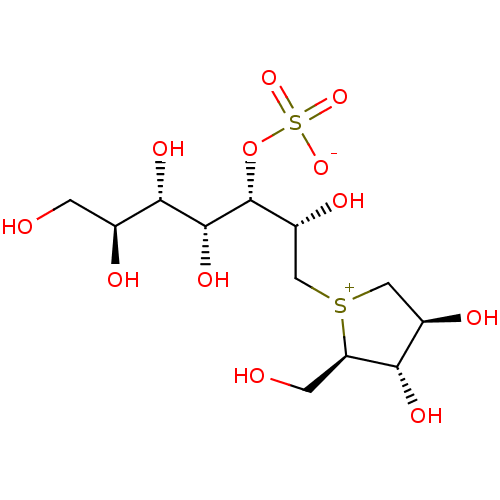
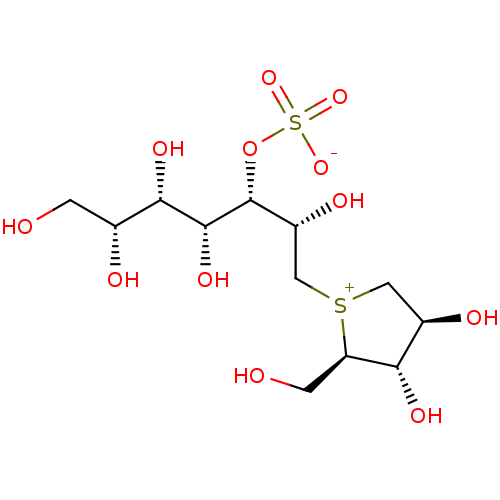
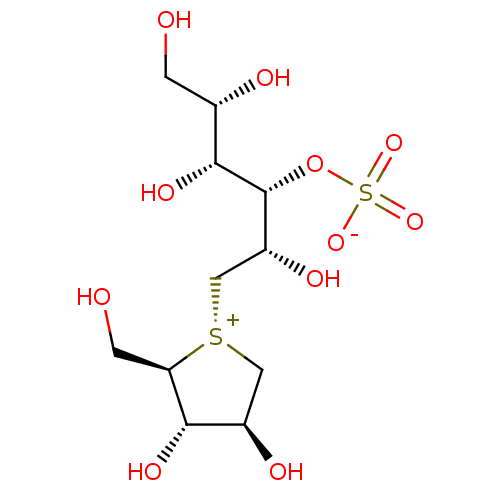
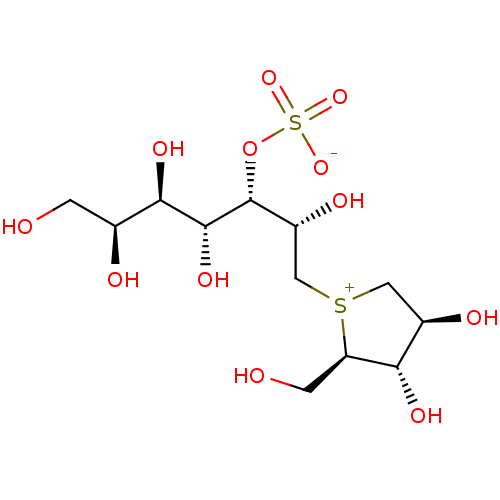
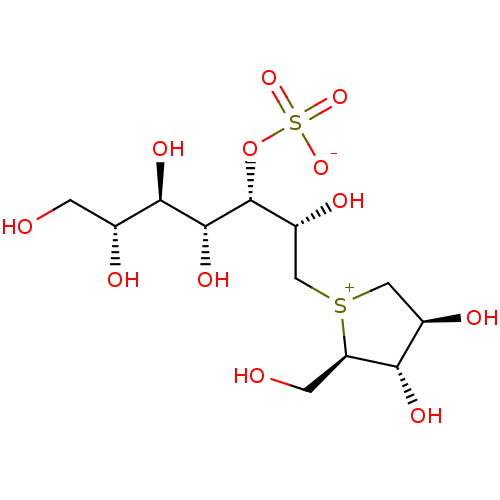
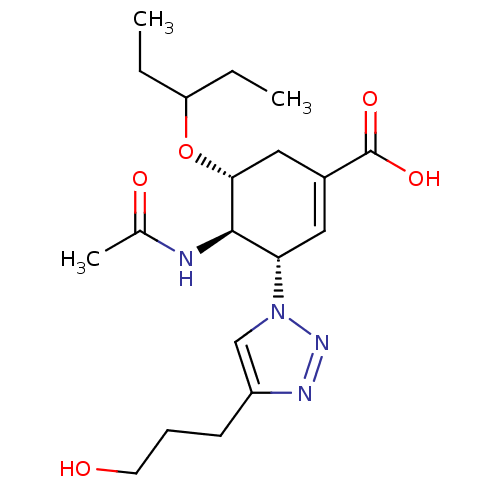
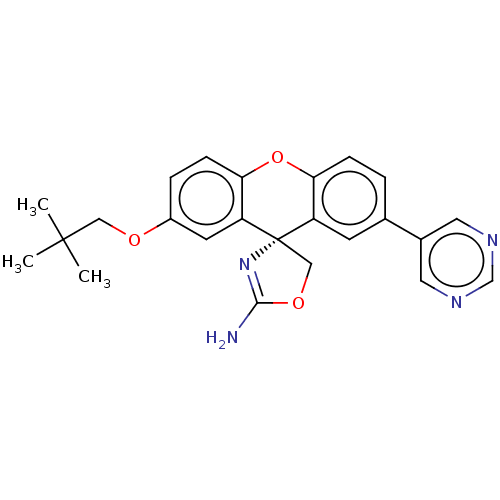
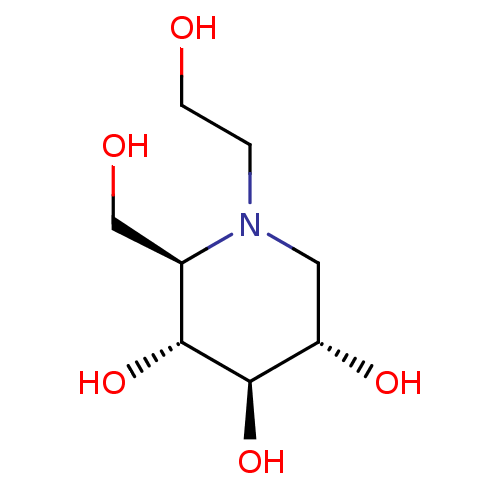
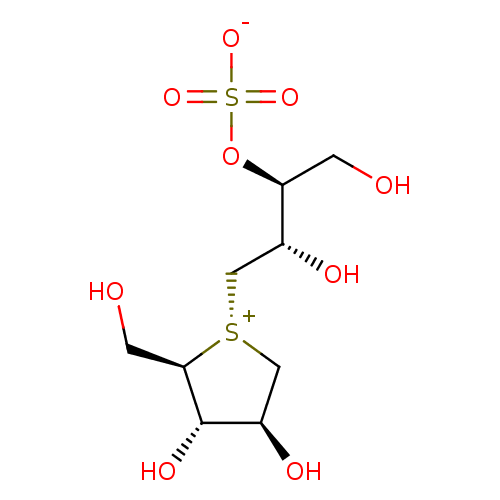
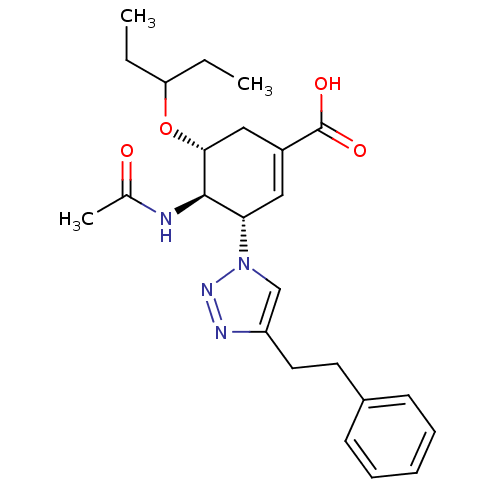
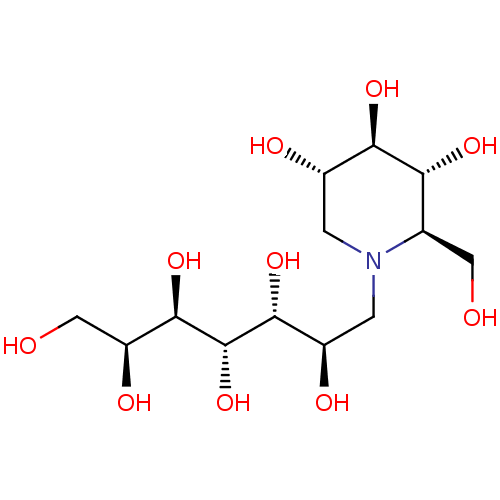
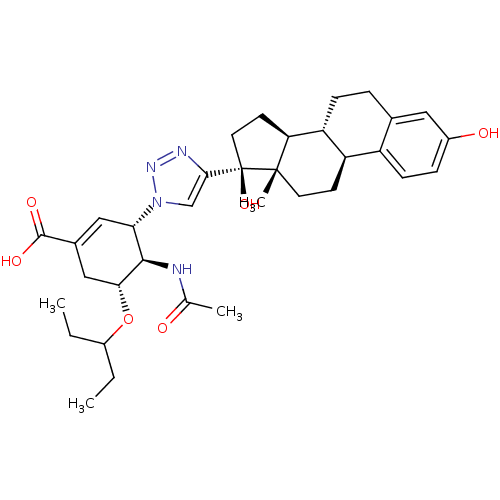
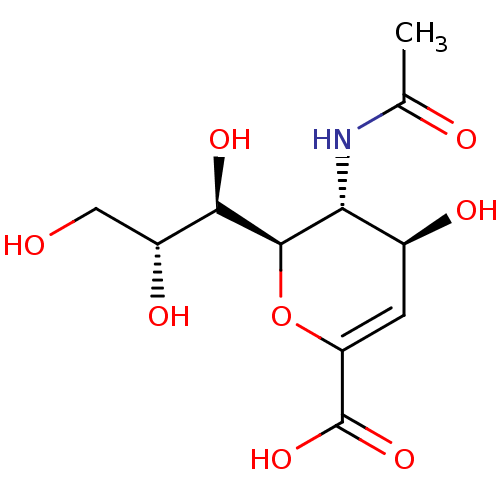
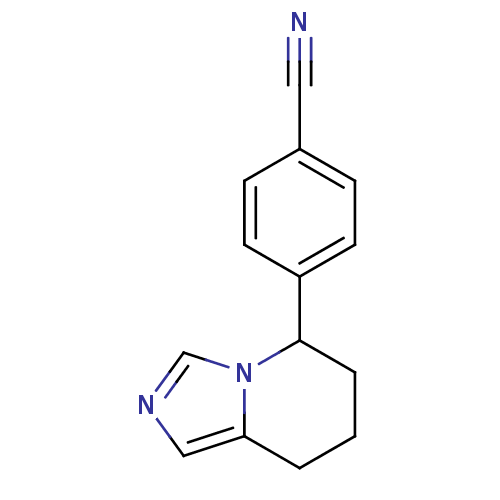
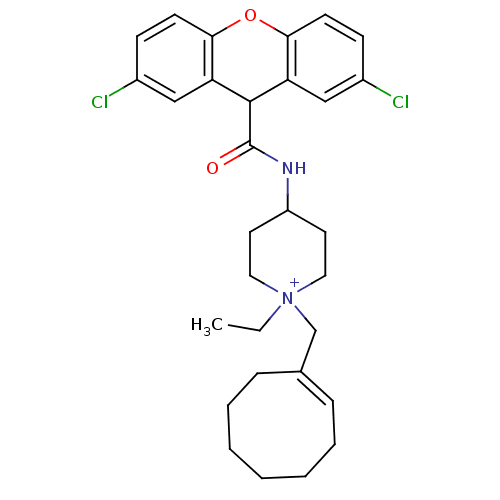
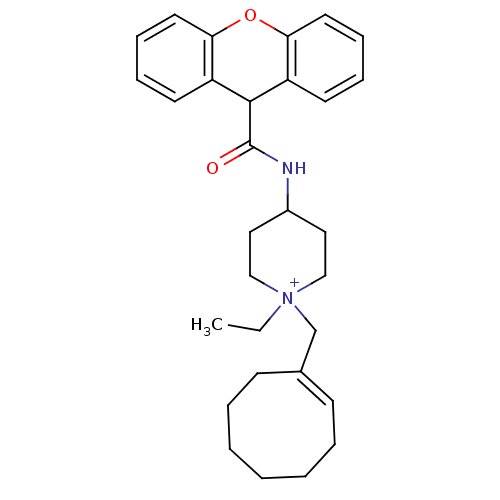
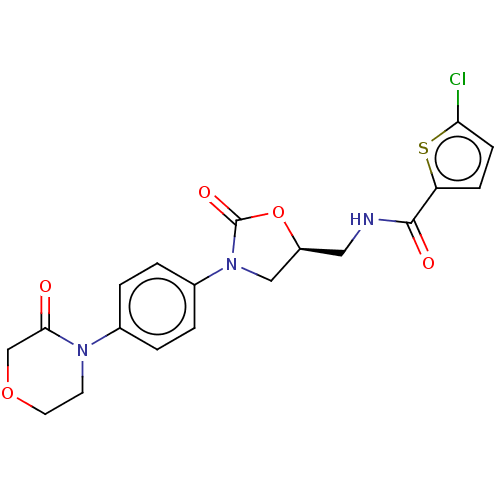
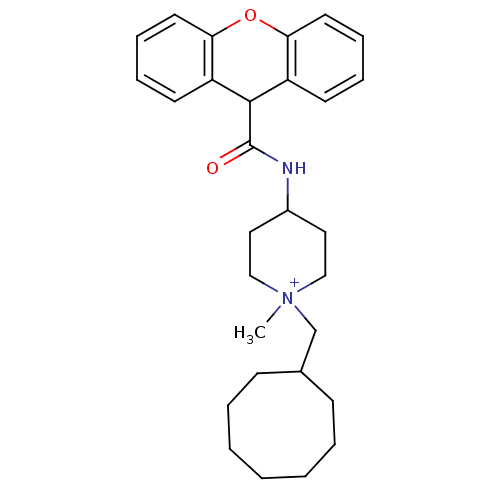
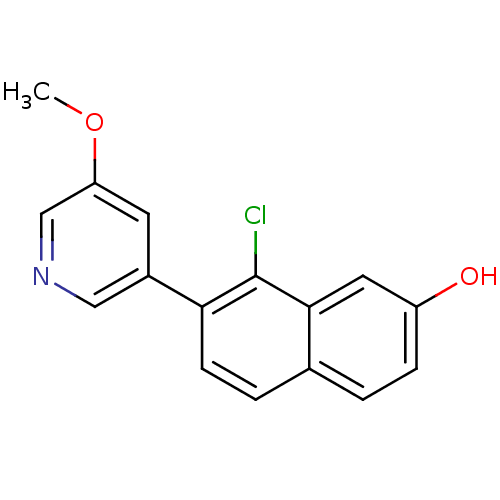
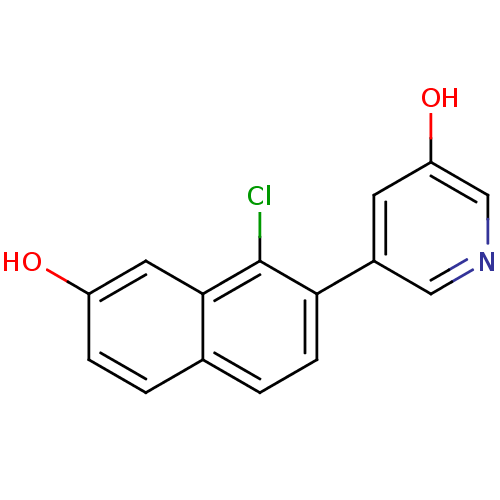
Affinity DataKi: 0.160nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibition of human N-terminal maltase-glucoamylaseMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 72nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domainMore data for this Ligand-Target Pair
Affinity DataKi: 103nMAssay Description:Inhibition of human recombinant C-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domainMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domainMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domainMore data for this Ligand-Target Pair
Affinity DataKi: 302nMAssay Description:Inhibition of human recombinant N-terminal Sucrase-isomaltase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 460nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of human N-terminal maltase-glucoamylaseMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of human recombinant N-terminal domain of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of human recombinant N-terminal maltase-glucoamylase by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Ciimar
Curated by ChEMBL
Ciimar
Curated by ChEMBL
Affinity DataKi: 660nMAssay Description:Binding affinity to human ERG assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.80E+3nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 5.90E+3nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of influenza A nuraminidase N1More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Universidade Do Porto (Cequimed-Up)
Curated by ChEMBL
Affinity DataIC50: 4.59nMAssay Description:Inhibition of human factor 10a assessed as CBS 31.39 substrate hydrolysis by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of full length recombinant human POP expressed in Escherichia coli BL21 cells using Z-Gly-Pro-AMC as substrate measured for 5 minsMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPHMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human CCR1 transfected in CHO cells incubated for 1 hr in presence of 125I-chemokine by radioactivity based assayMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrateMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Saarland University & Helmholtz Institute For Pharmaceutical Research Saarland (Hips)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPHMore data for this Ligand-Target Pair

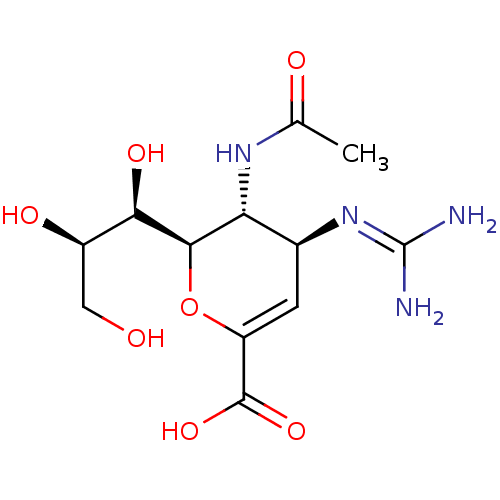
 3D Structure (crystal)
3D Structure (crystal)