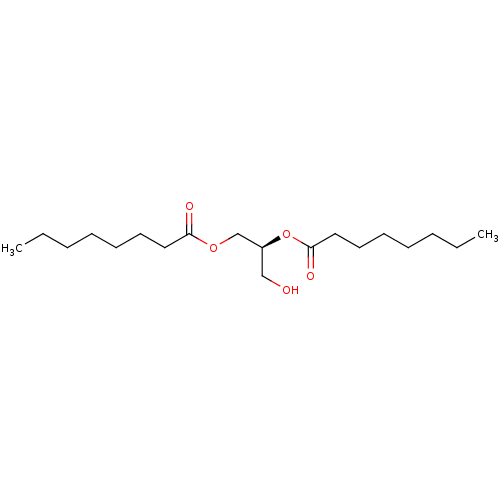
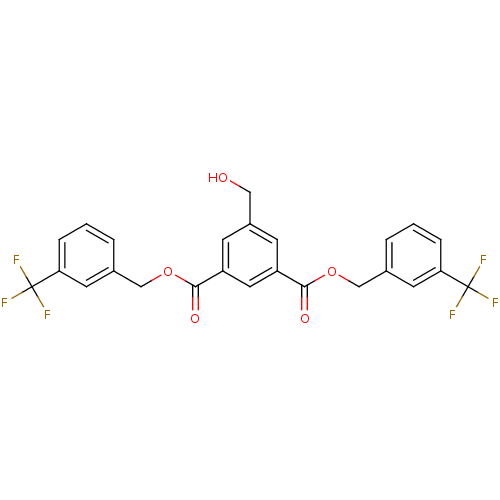
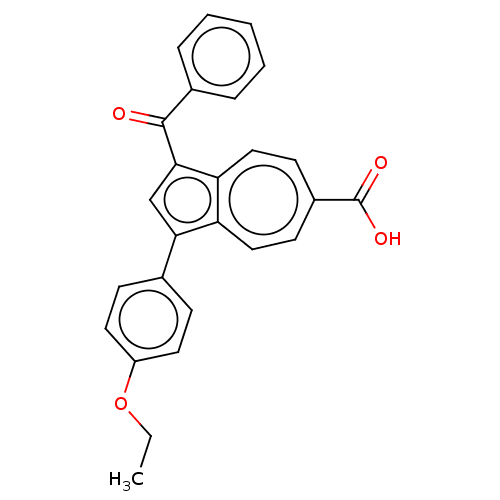
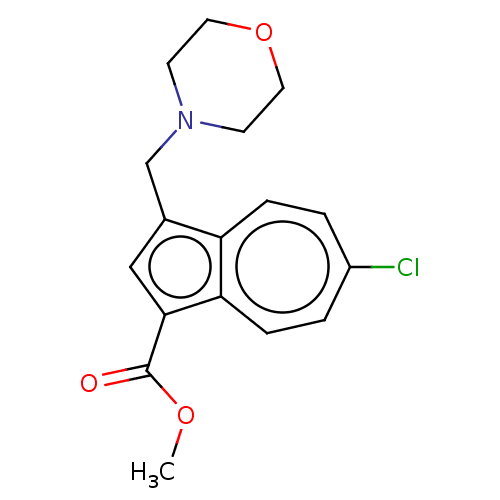
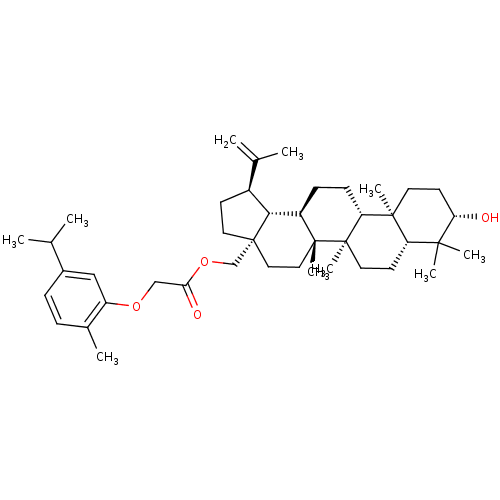
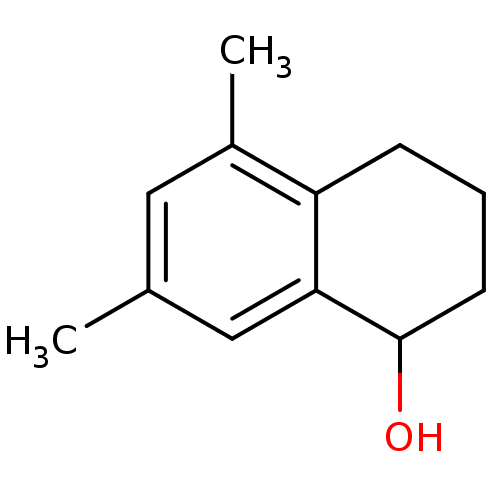
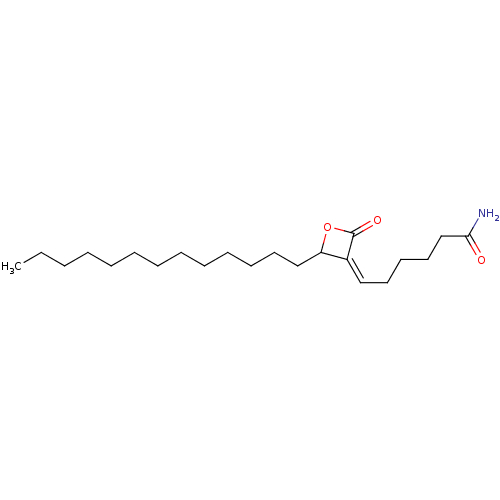
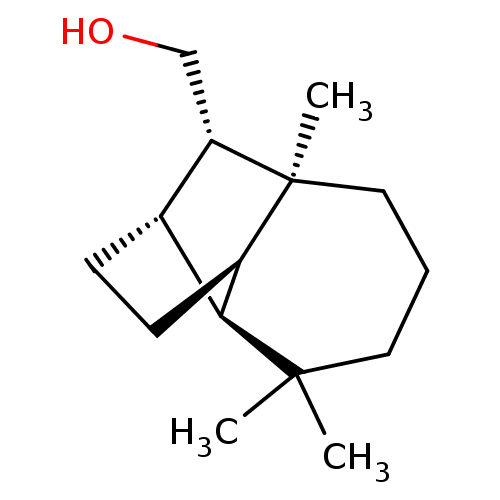
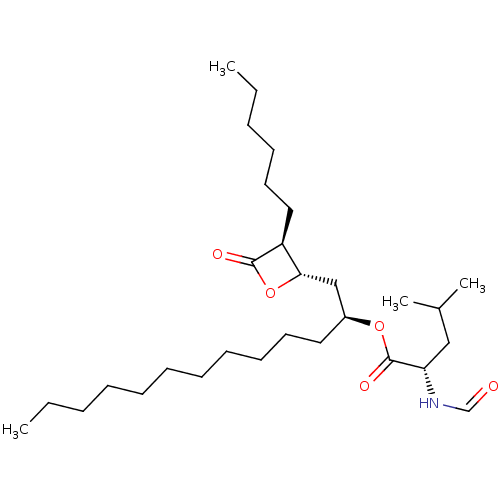
Affinity DataKi: 0.440nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
Affinity DataKi: 18.4nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 205nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 319nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 340nMAssay Description:Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
Affinity DataKi: 529nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 590nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 661nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 800nMAssay Description:Inhibition of Clostridium BoNT/A protease light chainMore data for this Ligand-Target Pair
Affinity DataKi: 814nMAssay Description:Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
Affinity DataKi: 915nMAssay Description:Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.08E+3nMAssay Description:Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
Affinity DataKi: 1.13E+3nMAssay Description:Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
Affinity DataKi: 1.69E+3nMAssay Description:Displacement of [3H]diprenorphine from human DOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m...More data for this Ligand-Target Pair
TargetOrexin/Hypocretin receptor type 1(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
Affinity DataKi: 4.30E+3nMAssay Description:Antagonist activity at human OX2 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
TargetOrexin/Hypocretin receptor type 1(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
TargetOrexin/Hypocretin receptor type 1(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataKi: 5.40E+3nMAssay Description:Antagonist activity at human OX1 receptor expressed in CHOK1 cells assessed as inhibition of orexin-A-induced calcium accumulation preincubated for 3...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of Clostridium BoNT/A protease light chainMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.43E+4nMAssay Description:Inhibition of Clostridium BoNT/A protease light chainMore data for this Ligand-Target Pair
Affinity DataKi: 2.18E+4nMAssay Description:Inhibition of human recombinant UGT2B17 assessed as reduction of scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 5.78E+4nMAssay Description:Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 1.01E+5nMAssay Description:Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+5nMAssay Description:Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 1.90E+5nMAssay Description:Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 1.92E+5nMAssay Description:Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 2.01E+5nMAssay Description:Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 2.58E+5nMAssay Description:Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 3.01E+5nMAssay Description:Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 4.27E+5nMAssay Description:Competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 6.08E+5nMAssay Description:Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 6.24E+5nMAssay Description:Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
Affinity DataKi: 1.44E+6nMAssay Description:Non competitive inhibition of UGT2B17-catalyzed scopoletin glucuronidationMore data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of ABHD16A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of human UGT2B7 by substrate-independent inhibition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Reversible inhibition of human ABHD16A expressed in HEK293 cells preincubated for 30 mins followed by 40 fold compound dilution and subsequent 1-LG s...More data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of ABHD16A (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 99nMAssay Description:Inhibition of human ABHD16A expressed in HEK293 cells preincubated for 30 mins followed by 1-LG substrate addition and measured after 90 to 120 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of ABHD16A (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylserine lipase ABHD16A(Homo sapiens (Human))
University Of Helsinki
Curated by ChEMBL
University Of Helsinki
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of ABHD16A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidationMore data for this Ligand-Target Pair

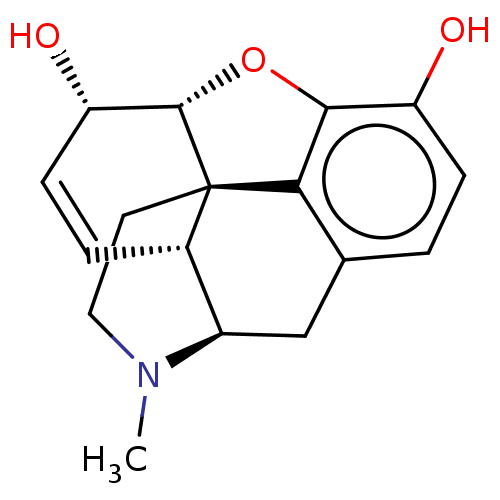
 3D Structure (crystal)
3D Structure (crystal)