Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bromodomain-containing protein 3
Ligand
BDBM50365262
Substrate
n/a
Meas. Tech.
ChEMBL_1903712 (CHEMBL4405934)
Kd
69±n/a nM
Citation
 Liu, Z; Chen, H; Wang, P; Li, Y; Wold, EA; Leonard, PG; Joseph, S; Brasier, AR; Tian, B; Zhou, J Discovery of Orally Bioavailable Chromone Derivatives as Potent and Selective BRD4 Inhibitors: Scaffold Hopping, Optimization, and Pharmacological Evaluation. J Med Chem 63:5242-5256 (2020) [PubMed] Article
Liu, Z; Chen, H; Wang, P; Li, Y; Wold, EA; Leonard, PG; Joseph, S; Brasier, AR; Tian, B; Zhou, J Discovery of Orally Bioavailable Chromone Derivatives as Potent and Selective BRD4 Inhibitors: Scaffold Hopping, Optimization, and Pharmacological Evaluation. J Med Chem 63:5242-5256 (2020) [PubMed] Article More Info.:
Target
Name:
Bromodomain-containing protein 3
Synonyms:
BRD3 | BRD3_HUMAN | Bromodomain and extra-terminal motif (BET) | Bromodomain-containing protein 3 | Bromodomain-containing protein 3 (BRD3) | KIAA0043 | RING3-like protein | RING3L
Type:
Protein
Mol. Mass.:
79571.81
Organism:
Homo sapiens (Human)
Description:
Q15059
Residue:
726
Sequence:
MSTATTVAPAGIPATPGPVNPPPPEVSNPSKPGRKTNQLQYMQNVVVKTLWKHQFAWPFYQPVDAIKLNLPDYHKIIKNPMDMGTIKKRLENNYYWSASECMQDFNTMFTNCYIYNKPTDDIVLMAQALEKIFLQKVAQMPQEEVELLPPAPKGKGRKPAAGAQSAGTQQVAAVSSVSPATPFQSVPPTVSQTPVIAATPVPTITANVTSVPVPPAAAPPPPATPIVPVVPPTPPVVKKKGVKRKADTTTPTTSAITASRSESPPPLSDPKQAKVVARRESGGRPIKPPKKDLEDGEVPQHAGKKGKLSEHLRYCDSILREMLSKKHAAYAWPFYKPVDAEALELHDYHDIIKHPMDLSTVKRKMDGREYPDAQGFAADVRLMFSNCYKYNPPDHEVVAMARKLQDVFEMRFAKMPDEPVEAPALPAPAAPMVSKGAESSRSSEESSSDSGSSDSEEERATRLAELQEQLKAVHEQLAALSQAPVNKPKKKKEKKEKEKKKKDKEKEKEKHKVKAEEEKKAKVAPPAKQAQQKKAPAKKANSTTTAGRQLKKGGKQASASYDSEEEEEGLPMSYDEKRQLSLDINRLPGEKLGRVVHIIQSREPSLRDSNPDEIEIDFETLKPTTLRELERYVKSCLQKKQRKPFSASGKKQAAKSKEELAQEKKKELEKRLQDVSGQLSSSKKPARKEKPGSAPSGGPSRLSSSSSSESGSSSSSGSSSDSSDSE
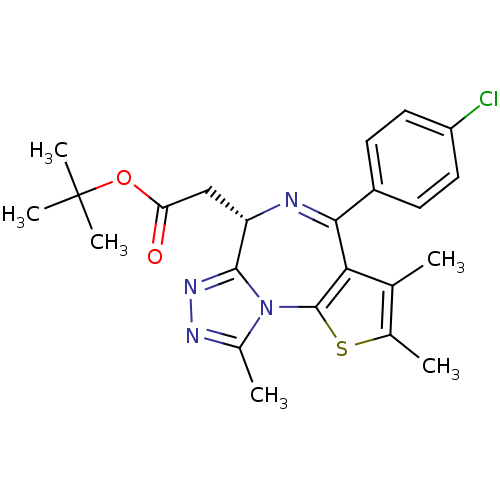
Inhibitor
Name:
BDBM50365262
Synonyms:
(+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10124009, Compound (S)-JQ1 | US10202360, Example JQ-1 | US10308662, Compound JQ-1 | US10407441, Compound (S)-JQ1 | US10617680, Example JQ-1 | US10881668, Compound JQ1 | US10925881, Name (S)-JQ1 | US11020380, Example JQ-1 | US11078188, Example (+)-JQ1 | US11279703, TABLE 6.180 | US11427593, Compound JQ1 | US11466034, Example (+)-JQ1 | US9320741, (S)-JQ1 | US9695172, JQ1
Type:
Small organic molecule
Emp. Form.:
C23H25ClN4O2S
Mol. Mass.:
456.988
SMILES:
Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14|