Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase BTK
Ligand
BDBM267819
Substrate
n/a
Meas. Tech.
ChEMBL_2061781 (CHEMBL4717034)
IC50
0.310000±n/a nM
Citation
 Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Boga, SB; Alhassan, AB; Yu, Y; Vaccaro, H; Liu, S; Yang, C; Wu, H; Cooper, A; de Man, J; Kaptein, A; Maloney, K; Hornak, V; Gao, YD; Fischmann, TO; Raaijmakers, H; Vu-Pham, D; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Zhang-Hoover, J; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Kim, R; Kozlowski, JA Discovery of 8-Amino-imidazo[1,5-a]pyrazines as Reversible BTK Inhibitors for the Treatment of Rheumatoid Arthritis. ACS Med Chem Lett 7:198-203 (2016) [PubMed] Article
Liu, J; Guiadeen, D; Krikorian, A; Gao, X; Wang, J; Boga, SB; Alhassan, AB; Yu, Y; Vaccaro, H; Liu, S; Yang, C; Wu, H; Cooper, A; de Man, J; Kaptein, A; Maloney, K; Hornak, V; Gao, YD; Fischmann, TO; Raaijmakers, H; Vu-Pham, D; Presland, J; Mansueto, M; Xu, Z; Leccese, E; Zhang-Hoover, J; Knemeyer, I; Garlisi, CG; Bays, N; Stivers, P; Brandish, PE; Hicks, A; Kim, R; Kozlowski, JA Discovery of 8-Amino-imidazo[1,5-a]pyrazines as Reversible BTK Inhibitors for the Treatment of Rheumatoid Arthritis. ACS Med Chem Lett 7:198-203 (2016) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein kinase BTK
Synonyms:
AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK)
Type:
Enzyme
Mol. Mass.:
76289.95
Organism:
Homo sapiens (Human)
Description:
Q06187
Residue:
659
Sequence:
MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEKITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEELRKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSSHRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDEYFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGKEGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELINYHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGKWRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANGCLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDFGLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYERFTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
Inhibitor
Name:
BDBM267819
Synonyms:
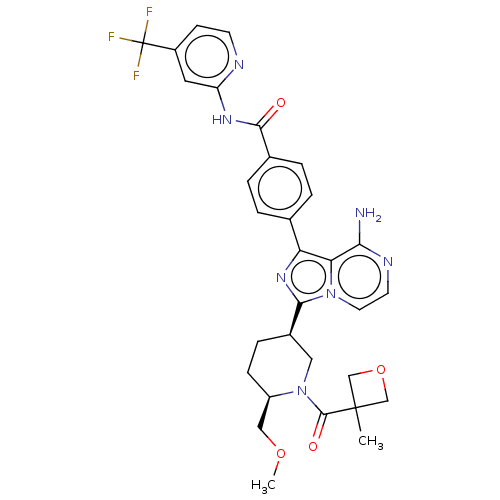
4-(8-amino-3-{(3S,6S)-6-(methoxymethyl)-1-[(3-methyloxetan-3-yl)carbonyl]piperidin-3-yl}imidazo[1,5-a]pyrazin-1-yl)-N-[4-(trifluoromethyl)pyridin-2-yl]benzamide | US9718828, Example, 429
Type:
Small organic molecule
Emp. Form.:
C31H32F3N7O4
Mol. Mass.:
623.6255
SMILES:
COC[C@H]1CC[C@H](CN1C(=O)C1(C)COC1)c1nc(-c2ccc(cc2)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r|