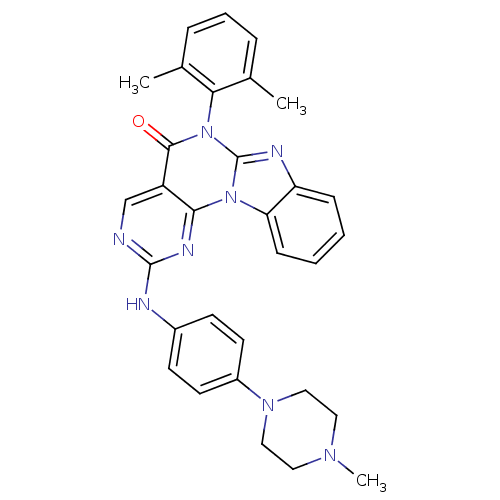
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine/threonine-protein kinase B-raf
Ligand
BDBM50374608
Substrate
n/a
Meas. Tech.
ChEMBL_469629 (CHEMBL934208)
IC50
540±n/a nM
Citation
 Martin, MW; Newcomb, J; Nunes, JJ; Boucher, C; Chai, L; Epstein, LF; Faust, T; Flores, S; Gallant, P; Gore, A; Gu, Y; Hsieh, F; Huang, X; Kim, JL; Middleton, S; Morgenstern, K; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, P; Tudor, Y; Turci, SM; Welcher, AA; Zack, D; Zhao, H; Zhu, L; Zhu, X; Ghiron, C; Ermann, M; Johnston, D; Saluste, CG Structure-based design of novel 2-amino-6-phenyl-pyrimido[5',4':5,6]pyrimido[1,2-a]benzimidazol-5(6H)-ones as potent and orally active inhibitors of lymphocyte specific kinase (Lck): synthesis, SAR, and in vivo anti-inflammatory activity. J Med Chem 51:1637-48 (2008) [PubMed] Article
Martin, MW; Newcomb, J; Nunes, JJ; Boucher, C; Chai, L; Epstein, LF; Faust, T; Flores, S; Gallant, P; Gore, A; Gu, Y; Hsieh, F; Huang, X; Kim, JL; Middleton, S; Morgenstern, K; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, P; Tudor, Y; Turci, SM; Welcher, AA; Zack, D; Zhao, H; Zhu, L; Zhu, X; Ghiron, C; Ermann, M; Johnston, D; Saluste, CG Structure-based design of novel 2-amino-6-phenyl-pyrimido[5',4':5,6]pyrimido[1,2-a]benzimidazol-5(6H)-ones as potent and orally active inhibitors of lymphocyte specific kinase (Lck): synthesis, SAR, and in vivo anti-inflammatory activity. J Med Chem 51:1637-48 (2008) [PubMed] Article More Info.:
Target
Name:
Serine/threonine-protein kinase B-raf
Synonyms:
B-RAF | B-Raf Protein Kinase | B-Raf proto-oncogene serine/threonine-protein kinase | BRAF | BRAF1 | BRAF_HUMAN | RAFB1 | p94 | v-Raf murine sarcoma viral oncogene homolog B1
Type:
Serine/threonine-protein kinase
Mol. Mass.:
84446.00
Organism:
Homo sapiens (Human)
Description:
P15056
Residue:
766
Sequence:
MAALSGGGGGGAEPGQALFNGDMEPEAGAGAGAAASSAADPAIPEEVWNIKQMIKLTQEHIEALLDKFGGEHNPPSIYLEAYEEYTSKLDALQQREQQLLESLGNGTDFSVSSSASMDTVTSSSSSSLSVLPSSLSVFQNPTDVARSNPKSPQKPIVRVFLPNKQRTVVPARCGVTVRDSLKKALMMRGLIPECCAVYRIQDGEKKPIGWDTDISWLTGEELHVEVLENVPLTTHNFVRKTFFTLAFCDFCRKLLFQGFRCQTCGYKFHQRCSTEVPLMCVNYDQLDLLFVSKFFEHHPIPQEEASLAETALTSGSSPSAPASDSIGPQILTSPSPSKSIPIPQPFRPADEDHRNQFGQRDRSSSAPNVHINTIEPVNIDDLIRDQGFRGDGGSTTGLSATPPASLPGSLTNVKALQKSPGPQRERKSSSSSEDRNRMKTLGRRDSSDDWEIPDGQITVGQRIGSGSFGTVYKGKWHGDVAVKMLNVTAPTPQQLQAFKNEVGVLRKTRHVNILLFMGYSTKPQLAIVTQWCEGSSLYHHLHIIETKFEMIKLIDIARQTAQGMDYLHAKSIIHRDLKSNNIFLHEDLTVKIGDFGLATVKSRWSGSHQFEQLSGSILWMAPEVIRMQDKNPYSFQSDVYAFGIVLYELMTGQLPYSNINNRDQIIFMVGRGYLSPDLSKVRSNCPKAMKRLMAECLKKKRDERPLFPQILASIELLARSLPKIHRSASEPSLNRAGFQTEDFSLYACASPKTPIQAGGYGAFPVH
Inhibitor
Name:
BDBM50374608
Synonyms:
CHEMBL410295
Type:
Small organic molecule
Emp. Form.:
C31H30N8O
Mol. Mass.:
530.6229
SMILES:
CN1CCN(CC1)c1ccc(Nc2ncc3c(n2)n2c(nc4ccccc24)n(-c2c(C)cccc2C)c3=O)cc1 |(-5.07,-9.29,;-3.87,-8.32,;-4.12,-6.8,;-2.92,-5.83,;-1.48,-6.38,;-1.24,-7.9,;-2.43,-8.87,;-.29,-5.41,;1.15,-5.96,;2.35,-4.99,;2.11,-3.47,;3.3,-2.5,;3.06,-.98,;4.25,-.01,;4.01,1.51,;2.57,2.06,;1.38,1.09,;1.62,-.43,;-.06,1.64,;-.3,3.16,;-1.82,3.4,;-2.52,2.03,;-4.01,1.63,;-4.41,.14,;-3.32,-.95,;-1.83,-.55,;-1.43,.94,;.89,4.13,;.65,5.65,;-.79,6.2,;-1.98,5.23,;-1.03,7.72,;.17,8.7,;1.6,8.15,;1.85,6.62,;3.28,6.07,;2.33,3.58,;3.53,4.55,;.67,-2.92,;-.53,-3.89,)|