Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lactase/phlorizin hydrolase
Ligand
BDBM50299749
Substrate
n/a
Meas. Tech.
ChEMBL_1435478 (CHEMBL3384693)
IC50
35000±n/a nM
Citation
 Ghisaidoobe, AT; van den Berg, RJ; Butt, SS; Strijland, A; Donker-Koopman, WE; Scheij, S; van den Nieuwendijk, AM; Koomen, GJ; van Loevezijn, A; Leemhuis, M; Wennekes, T; van der Stelt, M; van der Marel, GA; van Boeckel, CA; Aerts, JM; Overkleeft, HS Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J Med Chem 57:9096-104 (2014) [PubMed] Article
Ghisaidoobe, AT; van den Berg, RJ; Butt, SS; Strijland, A; Donker-Koopman, WE; Scheij, S; van den Nieuwendijk, AM; Koomen, GJ; van Loevezijn, A; Leemhuis, M; Wennekes, T; van der Stelt, M; van der Marel, GA; van Boeckel, CA; Aerts, JM; Overkleeft, HS Identification and development of biphenyl substituted iminosugars as improved dual glucosylceramide synthase/neutral glucosylceramidase inhibitors. J Med Chem 57:9096-104 (2014) [PubMed] Article More Info.:
Target
Name:
Lactase/phlorizin hydrolase
Synonyms:
LCT | LPH | LPH_HUMAN | Lactase-phlorizin hydrolase
Type:
PROTEIN
Mol. Mass.:
218576.40
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1435478
Residue:
1927
Sequence:
MELSWHVVFIALLSFSCWGSDWESDRNFISTAGPLTNDLLHNLSGLLGDQSSNFVAGDKDMYVCHQPLPTFLPEYFSSLHASQITHYKVFLSWAQLLPAGSTQNPDEKTVQCYRRLLKALKTARLQPMVILHHQTLPASTLRRTEAFADLFADYATFAFHSFGDLVGIWFTFSDLEEVIKELPHQESRASQLQTLSDAHRKAYEIYHESYAFQGGKLSVVLRAEDIPELLLEPPISALAQDTVDFLSLDLSYECQNEASLRQKLSKLQTIEPKVKVFIFNLKLPDCPSTMKNPASLLFSLFEAINKDQVLTIGFDINEFLSCSSSSKKSMSCSLTGSLALQPDQQQDHETTDSSPASAYQRIWEAFANQSRAERDAFLQDTFPEGFLWGASTGAFNVEGGWAEGGRGVSIWDPRRPLNTTEGQATLEVASDSYHKVASDVALLCGLRAQVYKFSISWSRIFPMGHGSSPSLPGVAYYNKLIDRLQDAGIEPMATLFHWDLPQALQDHGGWQNESVVDAFLDYAAFCFSTFGDRVKLWVTFHEPWVMSYAGYGTGQHPPGISDPGVASFKVAHLVLKAHARTWHHYNSHHRPQQQGHVGIVLNSDWAEPLSPERPEDLRASERFLHFMLGWFAHPVFVDGDYPATLRTQIQQMNRQCSHPVAQLPEFTEAEKQLLKGSADFLGLSHYTSRLISNAPQNTCIPSYDTIGGFSQHVNHVWPQTSSSWIRVVPWGIRRLLQFVSLEYTRGKVPIYLAGNGMPIGESENLFDDSLRVDYFNQYINEVLKAIKEDSVDVRSYIARSLIDGFEGPSGYSQRFGLHHVNFSDSSKSRTPRKSAYFFTSIIEKNGFLTKGAKRLLPPNTVNLPSKVRAFTFPSEVPSKAKVVWEKFSSQPKFERDLFYHGTFRDDFLWGVSSSAYQIEGAWDADGKGPSIWDNFTHTPGSNVKDNATGDIACDSYHQLDADLNMLRALKVKAYRFSISWSRIFPTGRNSSINSHGVDYYNRLINGLVASNIFPMVTLFHWDLPQALQDIGGWENPALIDLFDSYADFCFQTFGDRVKFWMTFNEPMYLAWLGYGSGEFPPGVKDPGWAPYRIAHAVIKAHARVYHTYDEKYRQEQKGVISLSLSTHWAEPKSPGVPRDVEAADRMLQFSLGWFAHPIFRNGDYPDTMKWKVGNRSELQHLATSRLPSFTEEEKRFIRATADVFCLNTYYSRIVQHKTPRLNPPSYEDDQEMAEEEDPSWPSTAMNRAAPWGTRRLLNWIKEEYGDIPIYITENGVGLTNPNTEDTDRIFYHKTYINEALKAYRLDGIDLRGYVAWSLMDNFEWLNGYTVKFGLYHVDFNNTNRPRTARASARYYTEVITNNGMPLAREDEFLYGRFPEGFIWSAASAAYQIEGAWRADGKGLSIWDTFSHTPLRVENDAIGDVACDSYHKIAEDLVTLQNLGVSHYRFSISWSRILPDGTTRYINEAGLNYYVRLIDTLLAASIQPQVTIYHWDLPQTLQDVGGWENETIVQRFKEYADVLFQRLGDKVKFWITLNEPFVIAYQGYGYGTAAPGVSNRPGTAPYIVGHNLIKAHAEAWHLYNDVYRASQGGVISITISSDWAEPRDPSNQEDVEAARRYVQFMGGWFAHPIFKNGDYNEVMKTRIRDRSLAAGLNKSRLPEFTESEKRRINGTYDFFGFNHYTTVLAYNLNYATAISSFDADRGVASIADRSWPDSGSFWLKMTPFGFRRILNWLKEEYNDPPIYVTENGVSQREETDLNDTARIYYLRTYINEALKAVQDKVDLRGYTVWSAMDNFEWATGFSERFGLHFVNYSDPSLPRIPKASAKFYASVVRCNGFPDPATGPHACLHQPDAGPTISPVRQEEVQFLGLMLGTTEAQTALYVLFSLVLLGVCGLAFLSYKYCKRSKQGKTQRSQQELSPVSSF
Inhibitor
Name:
BDBM50299749
Synonyms:
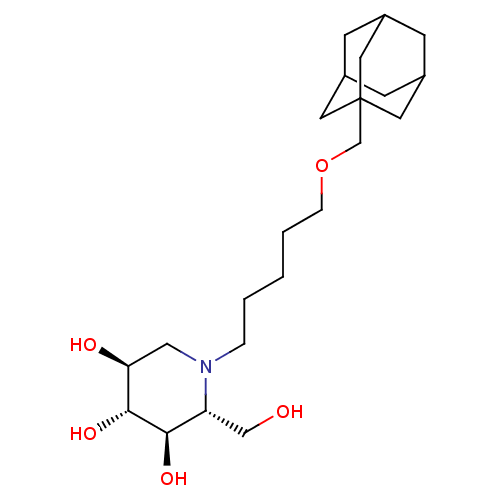
(2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]-2-hydroxymethyl-piperidine-3,4,5-triol | CHEMBL574645 | N-adamantanemethyloxypentyl-1-deoxynojirimycin
Type:
Small organic molecule
Emp. Form.:
C22H39NO5
Mol. Mass.:
397.5488
SMILES:
OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22|