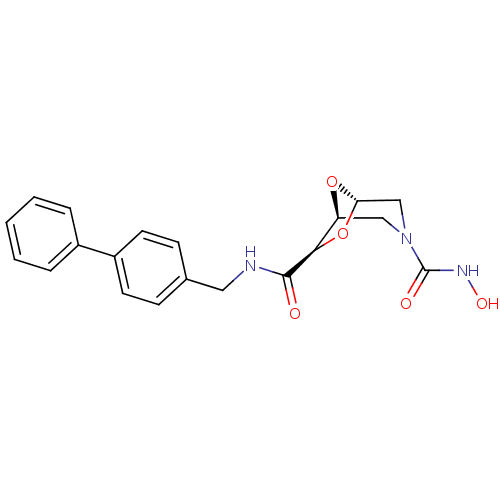
BDBM29018 (1S,5S,7R)-3-N-hydroxy-7-N-[(4-phenylphenyl)methyl]-6,8-dioxa-3-azabicyclo[3.2.1]octane-3,7-dicarboxamide::3-azadioxabicyclooctane derivative, 20
SMILES ONC(=O)N1C[C@H]2O[C@@H](C1)[C@@H](O2)C(=O)NCc1ccc(cc1)-c1ccccc1
InChI Key InChIKey=PPLDARNGJSQINK-OKZBNKHCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 29018
Found 7 hits for monomerid = 29018
Affinity DataIC50: 1.49E+5nM Kd: 1.54E+5nMpH: 7.0 T: 2°CAssay Description:The NMR experiments were performed to determine the binding affinity (KD) for the MMP-12 catalytic domain. The alteration of the chemical shifts indu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+6nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+6nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.78E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.65E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.49E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.13E+5nMpH: 7.0 T: 2°CAssay Description:The inhibition potency (IC50) was measured by fluorescent enzymatic assays. The compounds were evaluated for their ability to inhibit the hydrolysis ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)