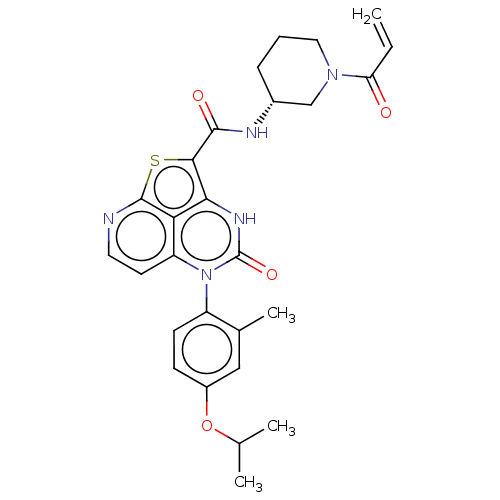
BDBM467759 (R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-isopropoxy-2- methylphenyl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide;::US10800792, Example 417::US10800792, Example 418
SMILES CC(C)Oc1ccc(c(C)c1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 467759
Found 4 hits for monomerid = 467759
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 337nMAssay Description:Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of BTK in C57Bl/6 mouse splenocyte assessed as reduction in anti-IgM-induced CD69 expression incubated for 1 hr followed anti-IgM stimulat...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL