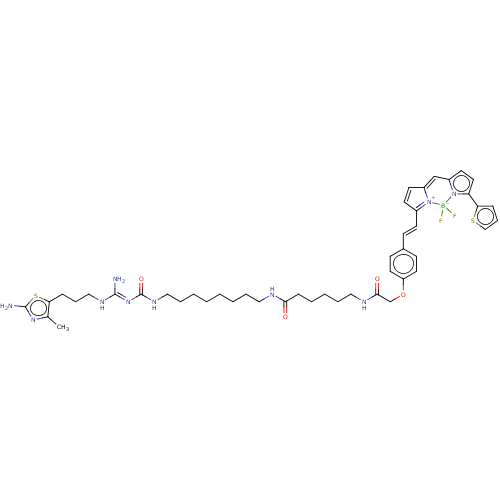
BDBM50586683 CHEMBL5078202
SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1nc(N)sc1CCCN\C(N)=N\C(=O)NCCCCCCCCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1
InChI Key InChIKey=JMFCZAMEBGGRSY-JWZFBPJHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50586683
Found 3 hits for monomerid = 50586683
Affinity DataKi: 30nMAssay Description:Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in Sf9 cell membranes by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 72nMAssay Description:Agonist activity at human H2 receptor expressed in Sf9 cell membranes co-exprssing GsalphaS assessed as stimulation of [35S]GTPgammaS binding incubat...More data for this Ligand-Target Pair
Affinity DataKd: 81nMAssay Description:Binding affinity to human H2R expressed in HEK293T-qs5-HA cells by flow cytometry analysisMore data for this Ligand-Target Pair