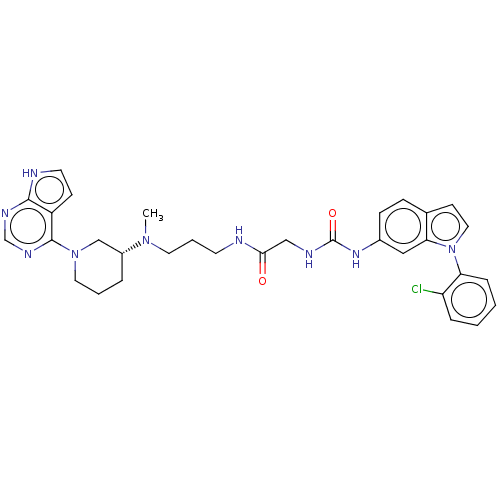
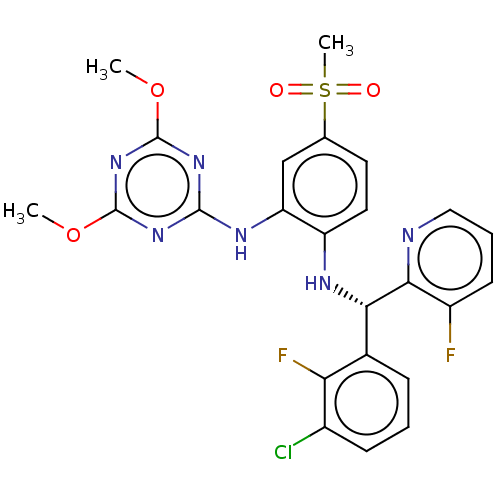
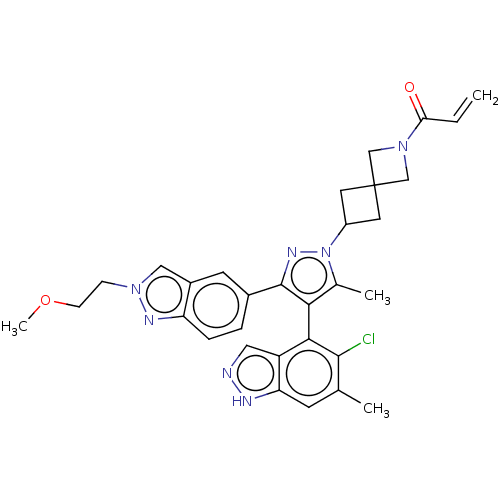
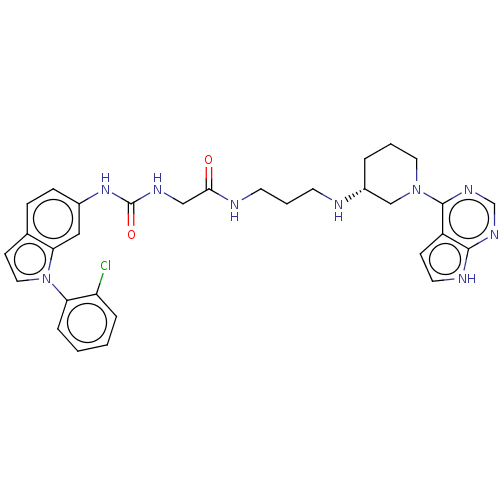
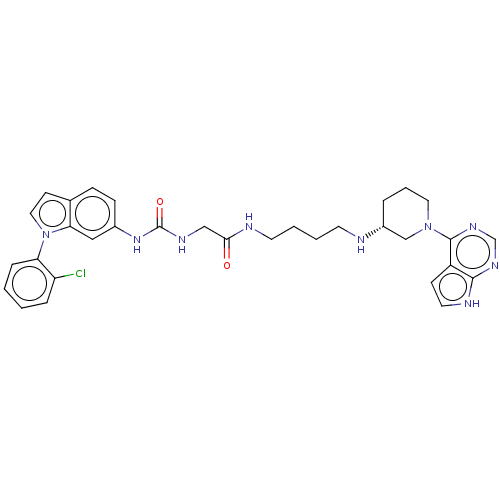
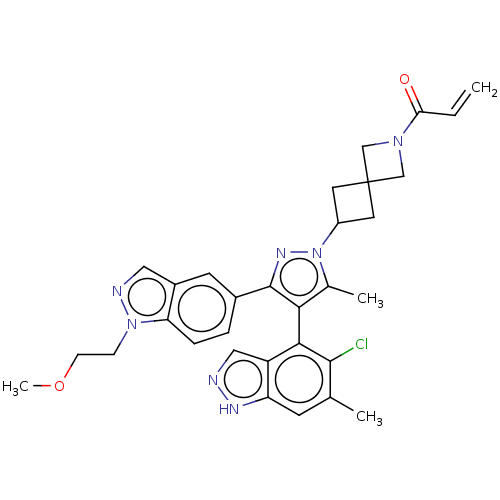
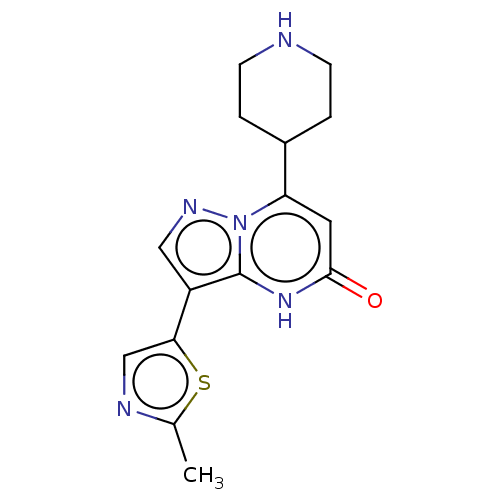
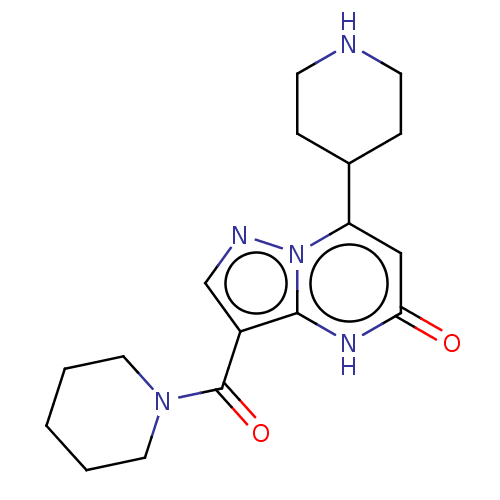
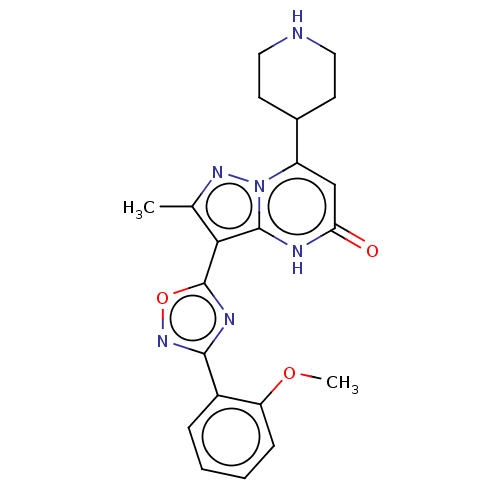
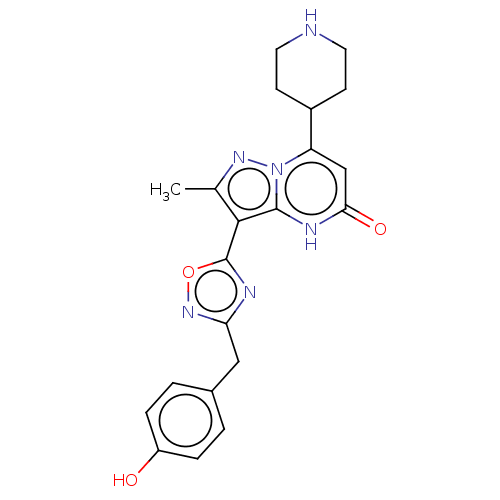
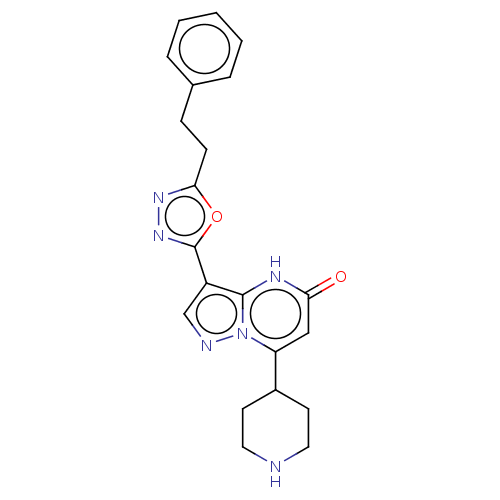
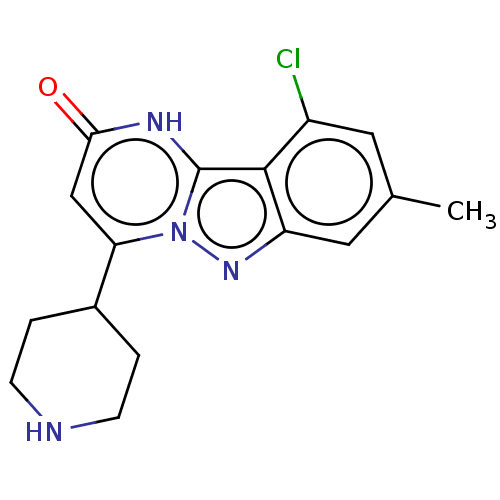
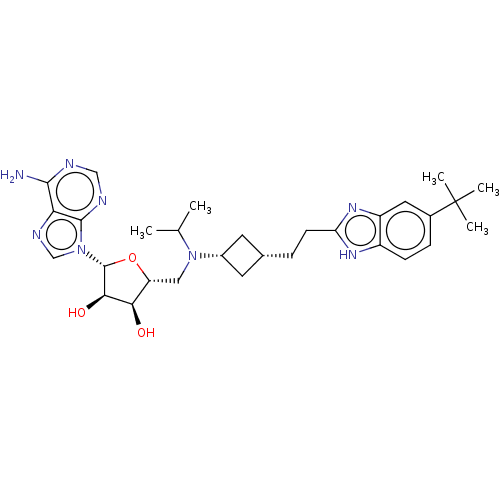
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.00200nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0120nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.360nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.590nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISAMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of 0.5 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 6 hrs by MSD assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 6nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 6 hrs by MSD assayMore data for this Ligand-Target Pair
Ligand Info
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8.20nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 8.70nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 9.70nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 6 hrs by MSD assayMore data for this Ligand-Target Pair
Ligand Info
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)