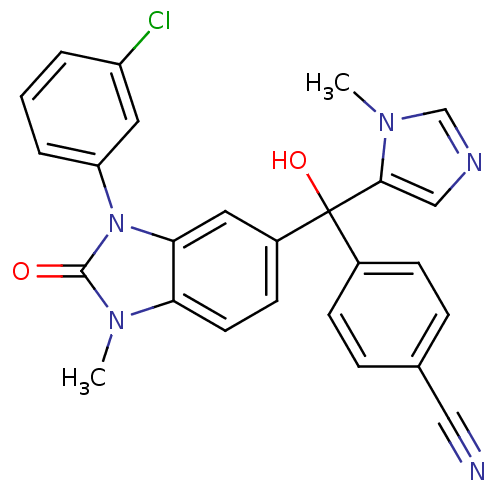
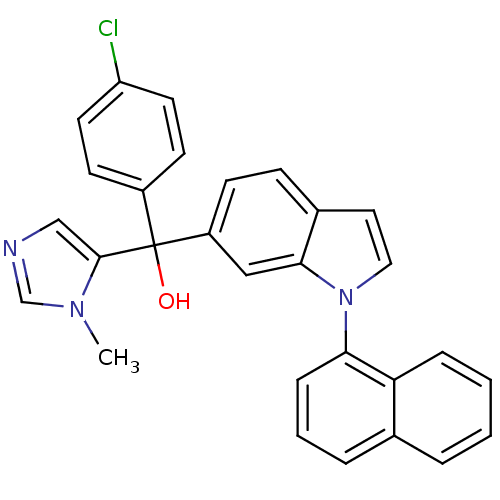
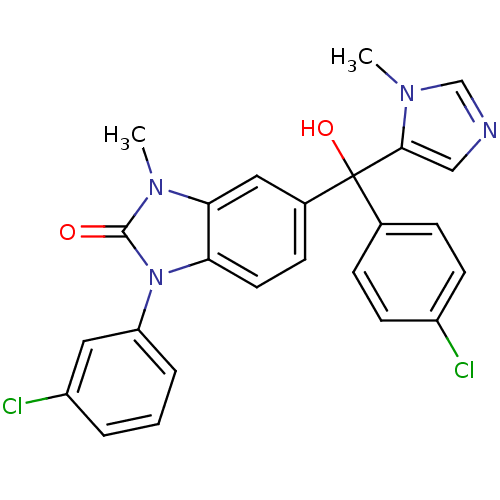
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 0.5nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
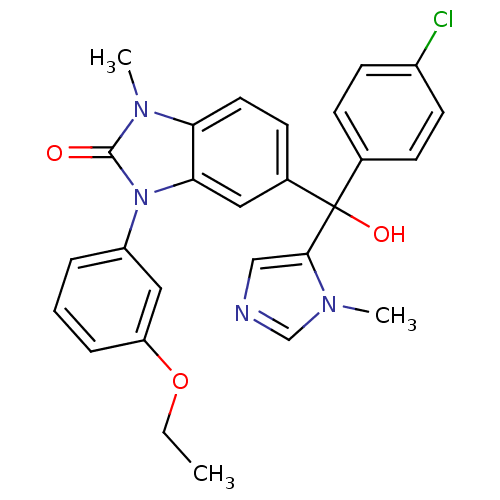
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 0.650nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
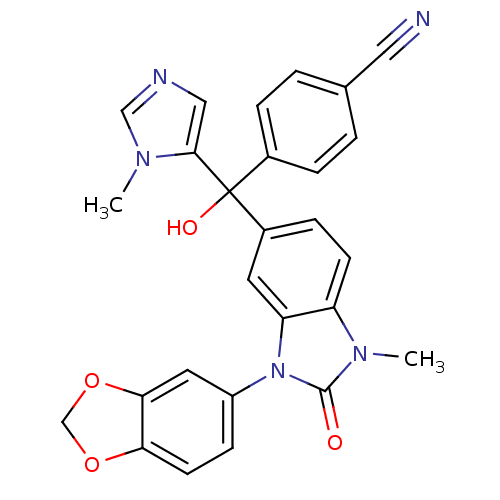
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.40nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
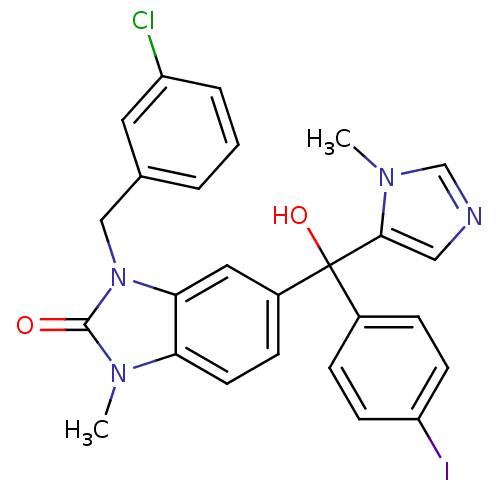
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 5.10nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 5.20nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 6.60nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 6.70nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 8nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 8.30nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 16nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 21nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 24nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 36nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 71nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 150nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 150nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 200nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.10E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.10E+3nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.20E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.80E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 2.90E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 5.60E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 7.50E+3nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: >1.00E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: >1.00E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: >1.00E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: >1.00E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: >1.00E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.10E+4nMAssay Description:The in vitro activity of compounds inhibiting GGTase-1 was determined by measuring the transfer of [3H]-geranylgeranylpyrophosphate to substrate Ha-R...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)