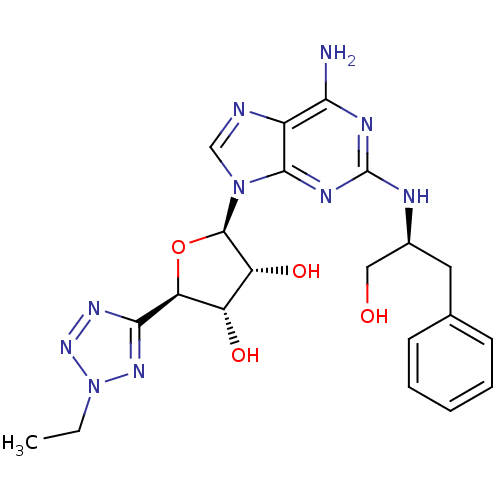
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Displacement of [3H]CGS21680 from human recombinant adenosine A2A receptor expressed in CHO cells after 60 mins by gamma counterMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Inhibition of [3H]ZM-241385 binding to human adenosine A2A receptor expressed in HeLa cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Binding affinity to human recombinant adenosine receptor A2aMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 8.80nMAssay Description:Potency against cAMP formation in CHO cells expressing recombinant human A2A receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Efficacy against phenylephrine precontracted tissue relaxation in rat aortaMore data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Agonist activity at adenosine receptor A2a in isolated rat aorta assessed as efficacyMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 9nMAssay Description:Agonist activity at adenosine receptor A2a (unknown origin) assessed as cAMP formationMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 1nMAssay Description:Agonist activity at human recombinant adenosine A2A receptor expressed in CHO cells assessed as induction of cyclic AMP productionMore data for this Ligand-Target Pair