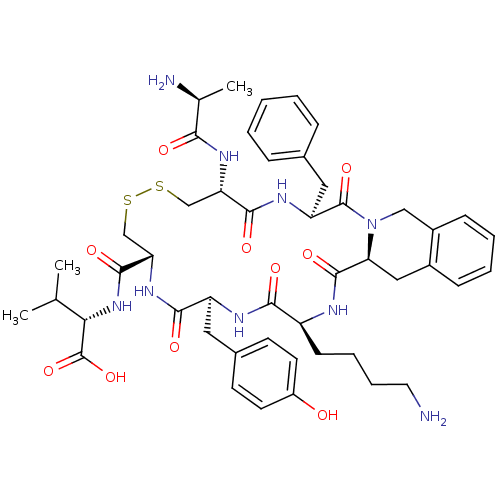
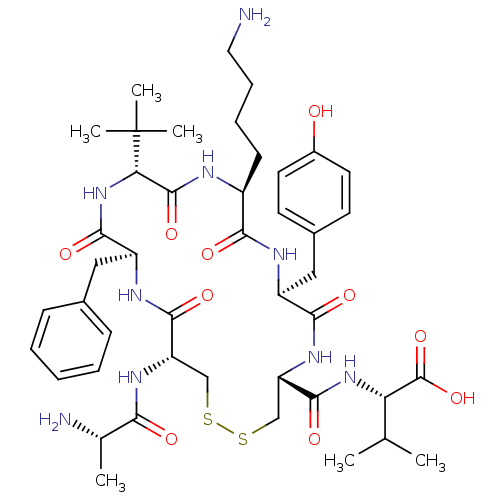
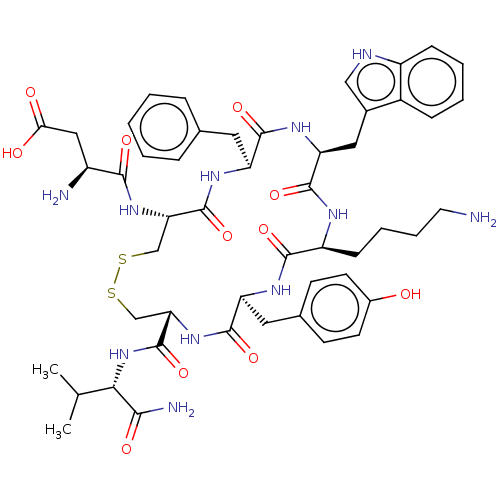
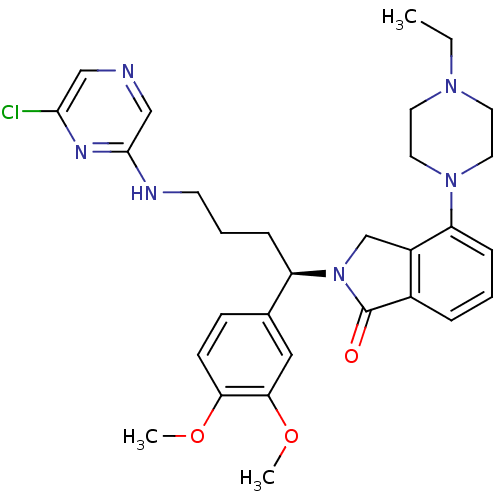
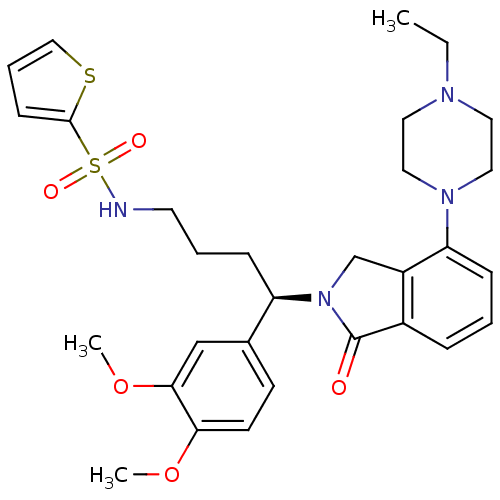
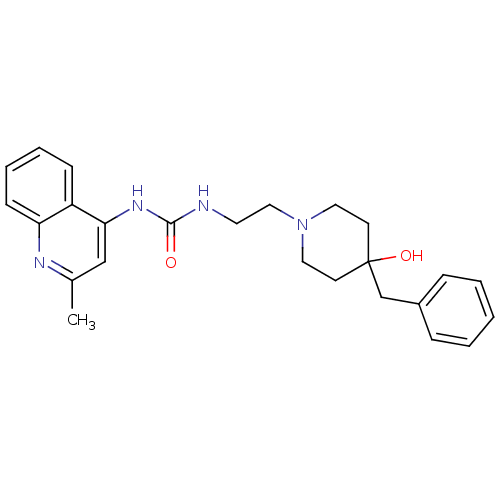
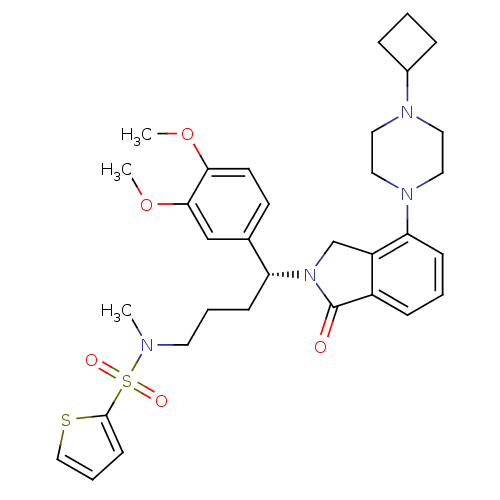
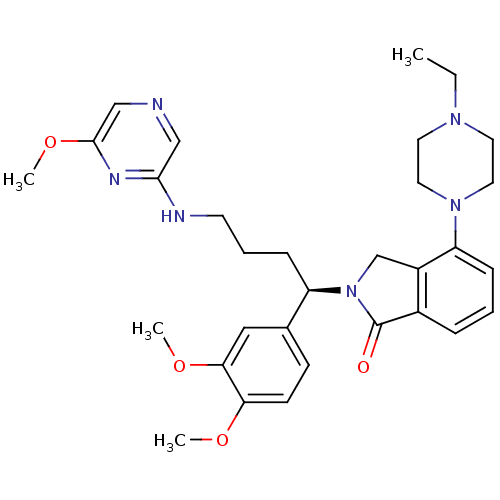
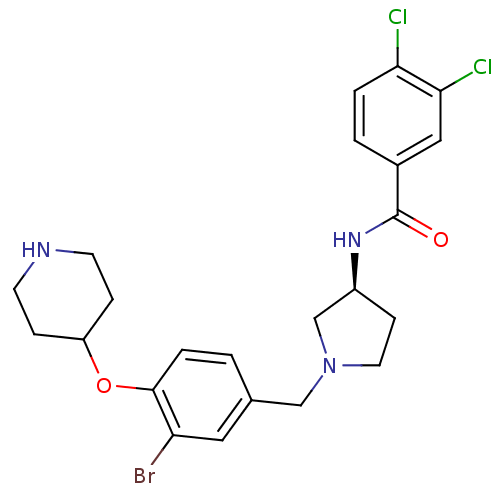
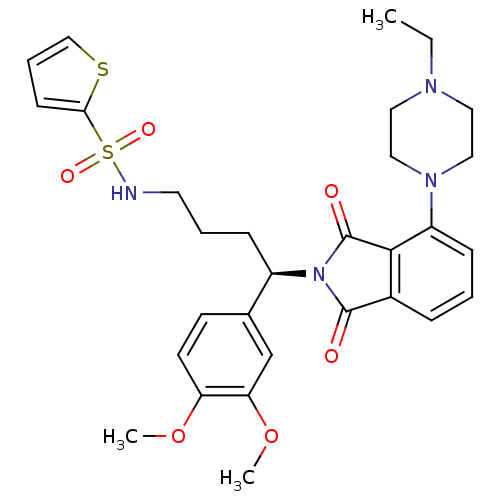
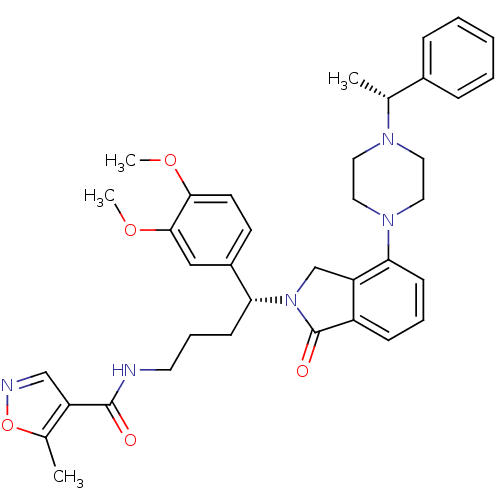
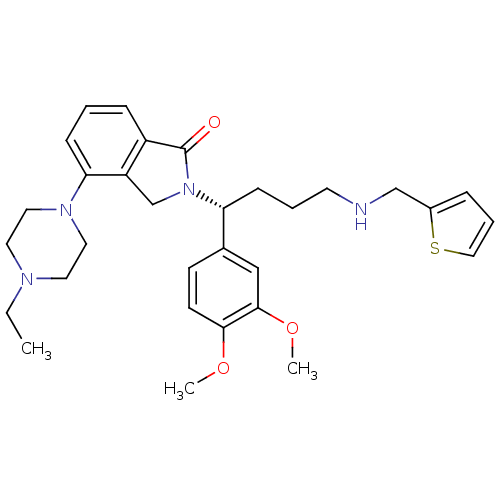
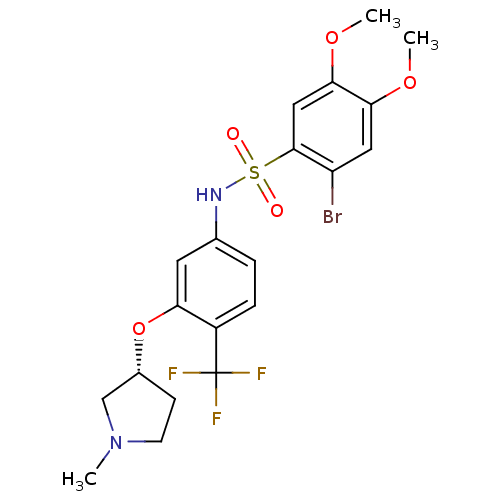
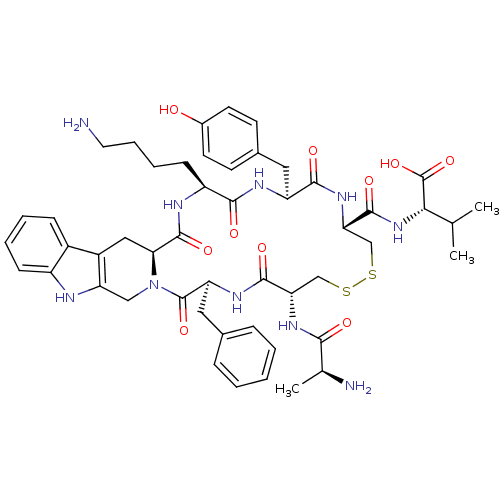
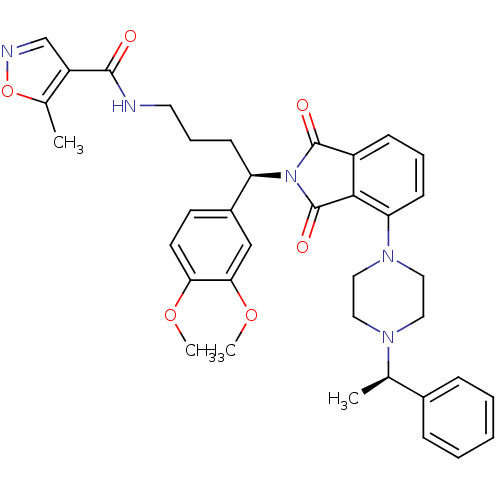
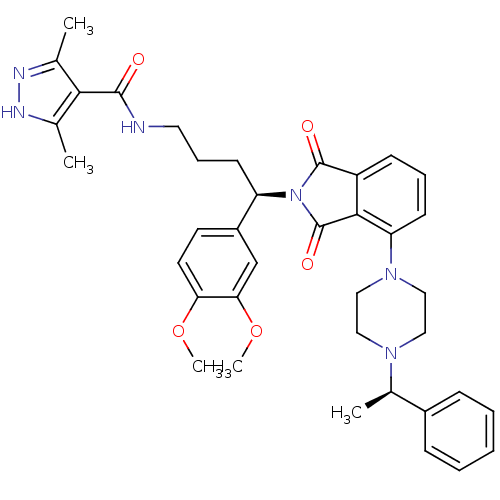
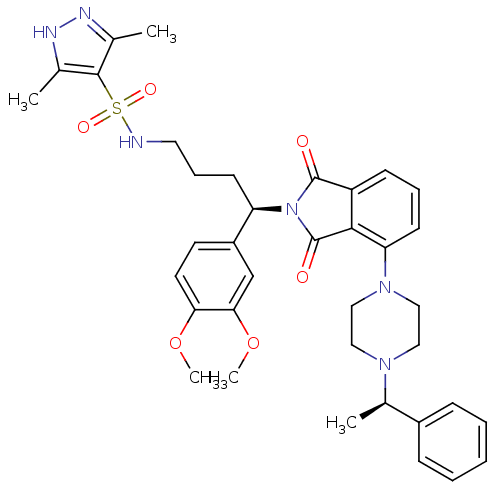
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
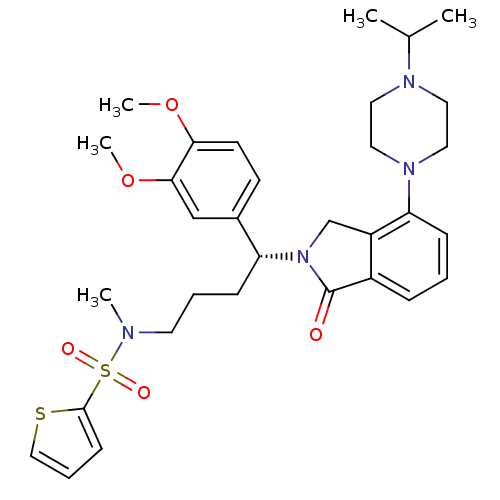
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
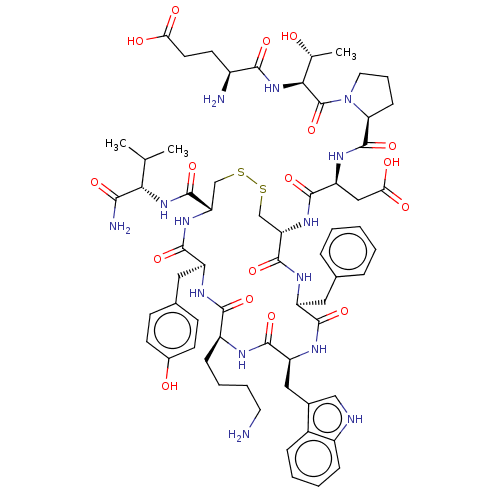
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
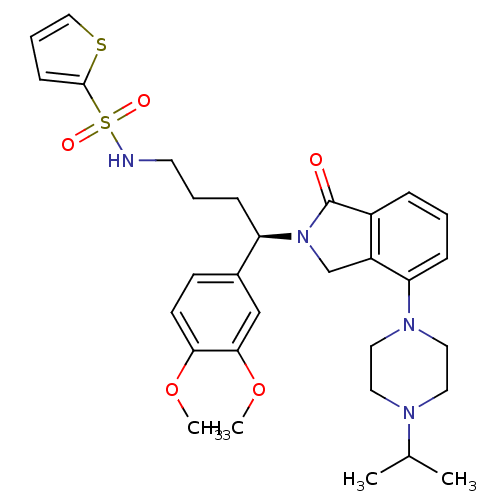
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >0nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Displacement of [125I-Tyr9]-U-II from human U-IIR expressed in HEK293-A cells incubated for 30 mins by gamma counting based competition radioligand b...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [125I-Tyr9]-U-II from human U-IIR expressed in HEK293-A cells incubated for 30 mins by gamma counting based competition radioligand b...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting methodMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting methodMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Binding affinity to human urotensin 2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-countingMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-countingMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of Eu-labelled UT2 from recombinant human UT2 receptor expressed in HEK293 cell membranes preincubated for 10 mins followed by Eu-labell...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Antagonist activity at urotensin 2 receptor in human RMS13 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobilization aft...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Antagonist activity at urotensin 2 receptor in human RMS13 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobilization aft...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Displacement of Eu-labelled UT2 from recombinant human UT2 receptor expressed in HEK293 cell membranes preincubated for 10 mins followed by Eu-labell...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Antagonist activity at rat UT receptor transfected in CHOK1 cells assessed as calcium mobilization by FLIPR methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of [125I]-Urotensin-2 from human GPR14 expressed in CHO cells after 90 mins by gamma counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of europium-labeled urotensin-II from human urotensin-2 receptor expressed in HEK293 cells by TRF assayMore data for this Ligand-Target Pair