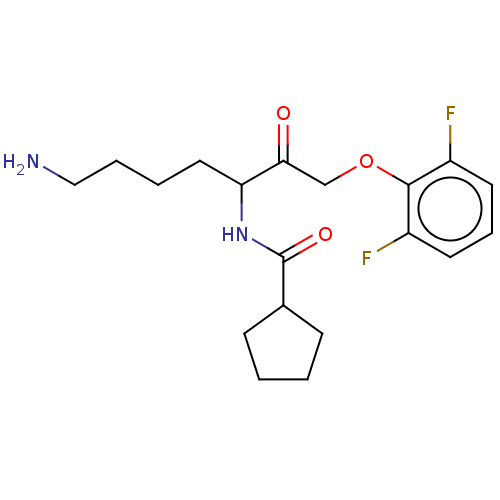
BDBM453289 US10730826, Compound 2a::US11325884, Compound 2a
SMILES NCCCCC(NC(=O)C1CCCC1)C(=O)COc1c(F)cccc1F
InChI Key InChIKey=DOHXJAQSKHNDLH-UHFFFAOYSA-N
Data 28 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 28 hits for monomerid = 453289
Found 28 hits for monomerid = 453289
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t...More data for this Ligand-Target Pair
Affinity DataIC50: 2.29E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair