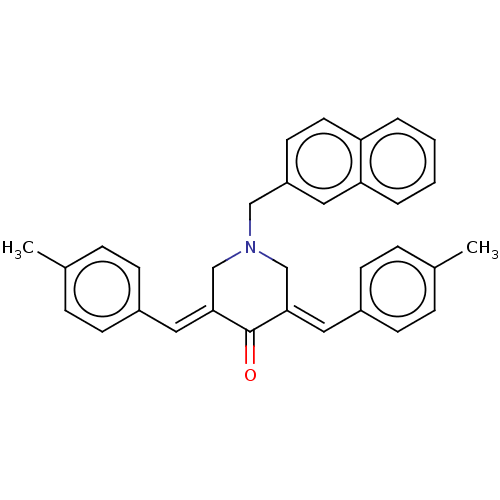
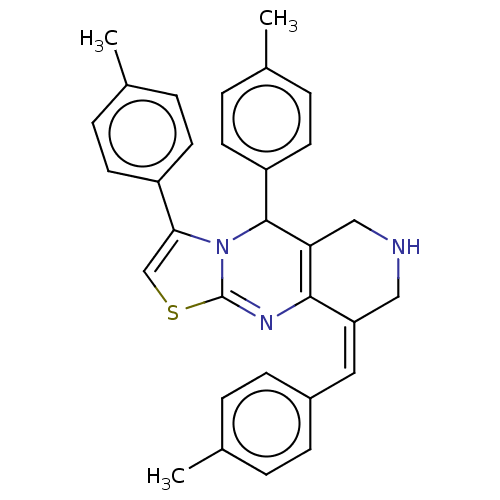
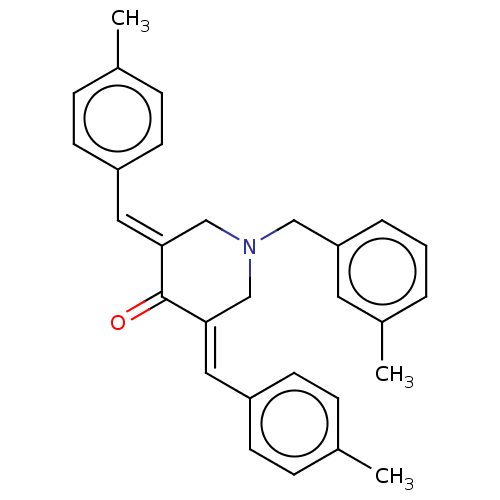
Affinity DataKi: 244nMAssay Description:Binding affinity to sigma2 receptor (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.22E+3nMAssay Description:Binding affinity to alpha2C receptor (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.26E+3nMAssay Description:Binding affinity to alpha1D receptor (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
University Of Kansas
Curated by ChEMBL
University Of Kansas
Curated by ChEMBL
Affinity DataKi: 3.41E+3nMAssay Description:Binding affinity to DAT receptor (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.56E+3nMAssay Description:Binding affinity to H1 receptor (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 2.25E+4nMAssay Description:Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 3.63E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 8.55E+4nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.01E+5nMAssay Description:Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.40E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 1.99E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 2.15E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataKi: 2.20E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis recombinant protein tyrosine phosphatase A by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 830nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 980nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.01E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.35E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.48E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.59E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 2.27E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 3.18E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
University Of Nebraska Medical Center
Curated by ChEMBL
University Of Nebraska Medical Center
Curated by ChEMBL
Affinity DataIC50: 3.22E+3nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as reduction in formation of 5-thio-2-nitrobenzoate from acetylthiocholine iodide preinc...More data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 1.10E+5nMAssay Description:Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 2.36E+5nMAssay Description:Non-competitive inhibition of Mycobacterium tuberculosis PTPA using p-nitrophenyl phosphate as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair

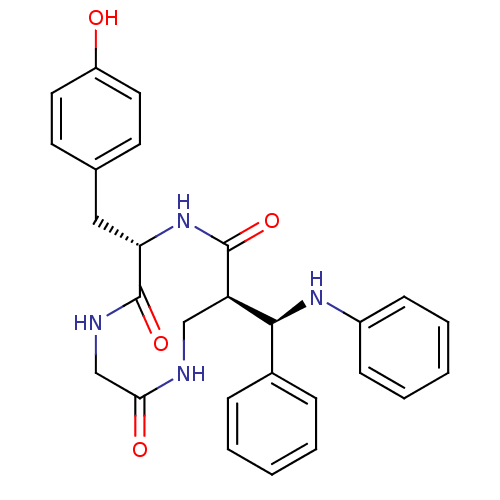
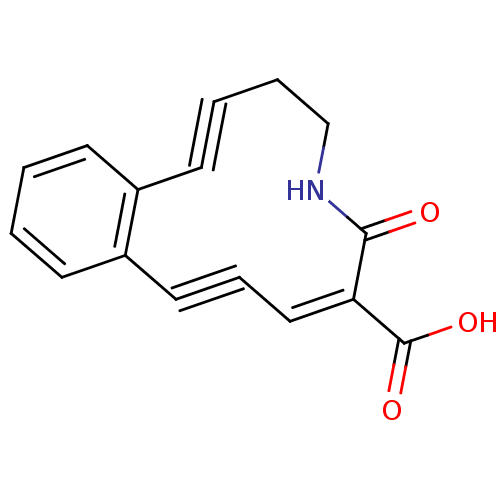
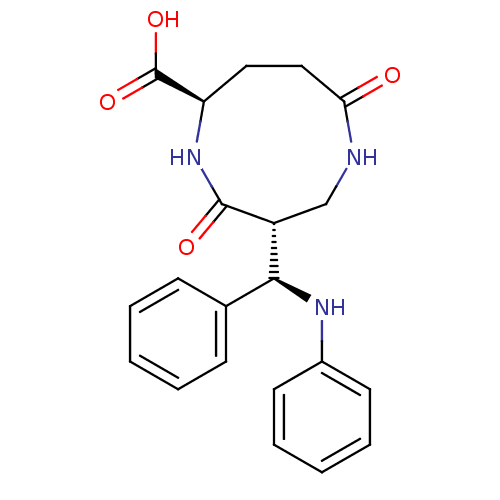
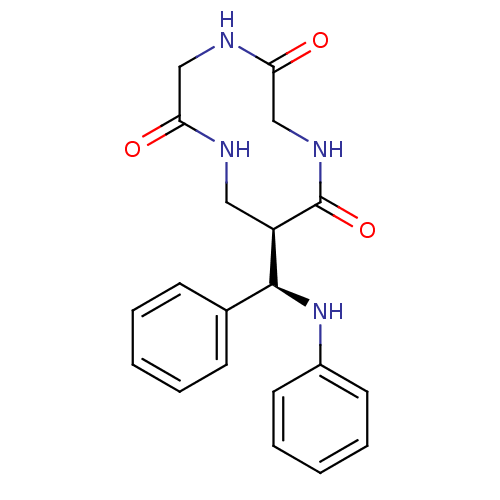




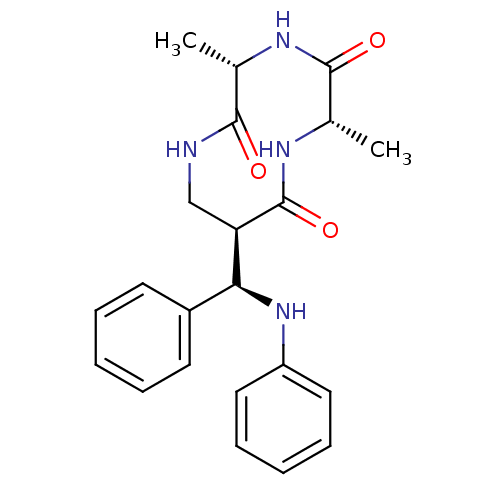


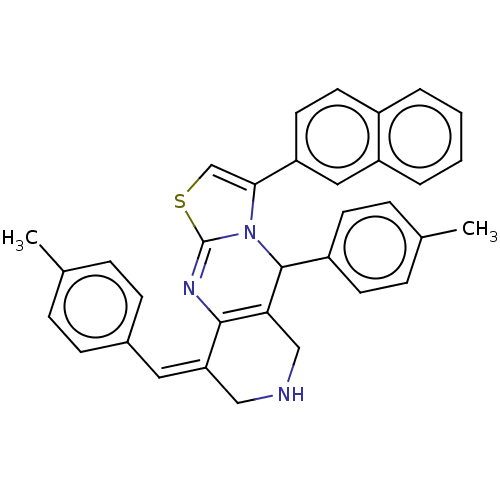
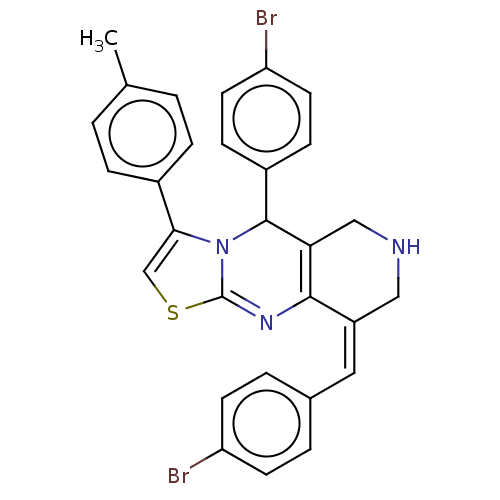
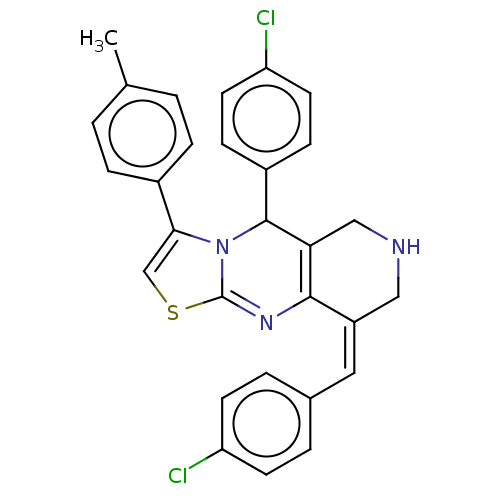
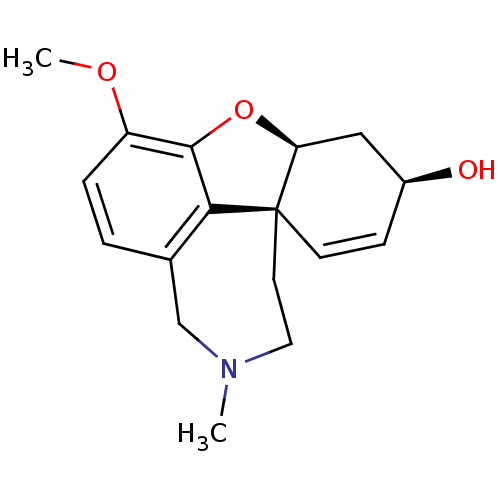
 3D Structure (crystal)
3D Structure (crystal)