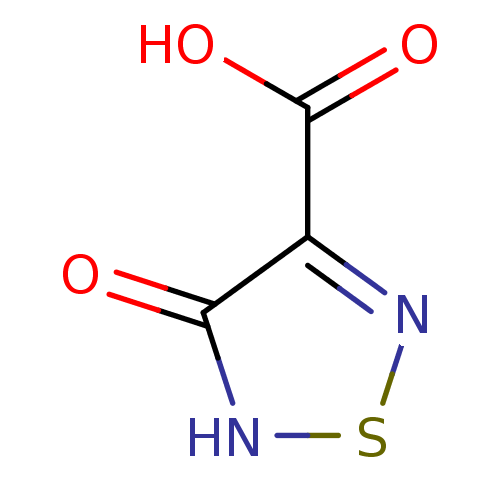
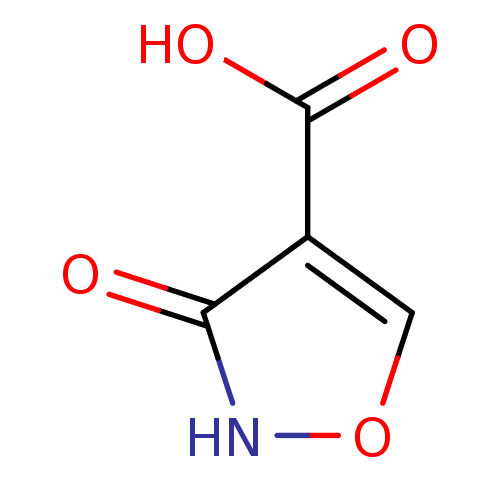
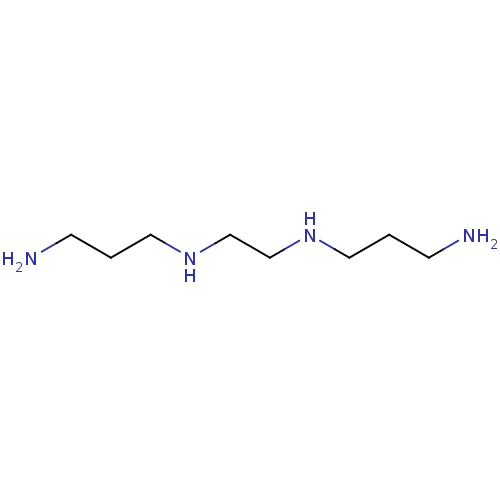
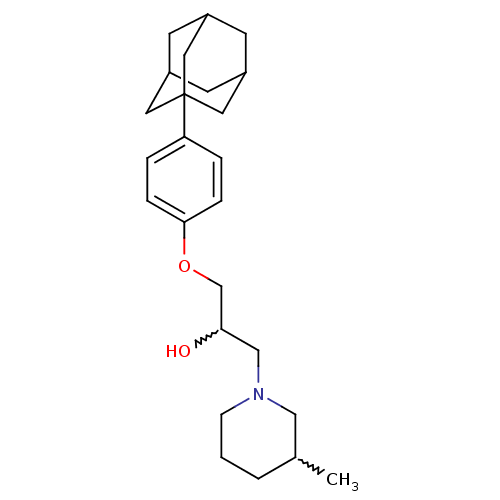
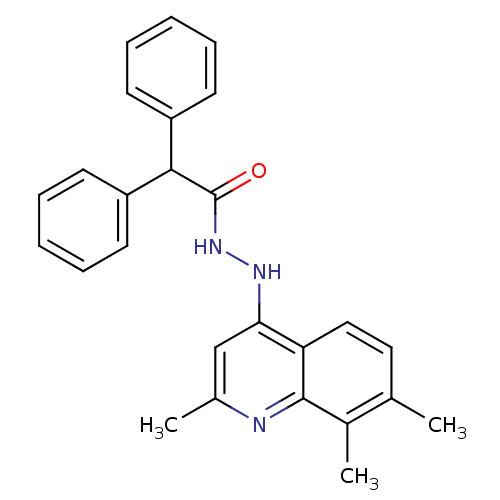
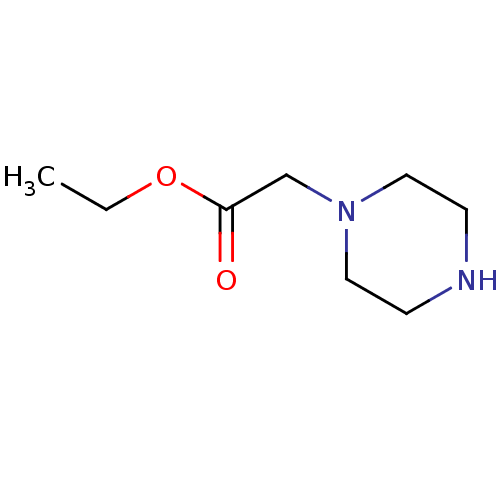
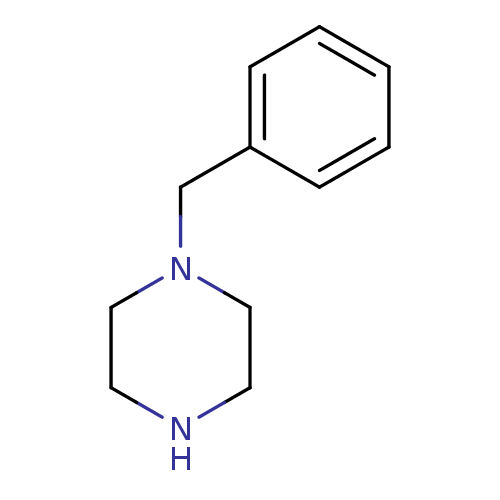
Affinity DataKi: 4.70E+3nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
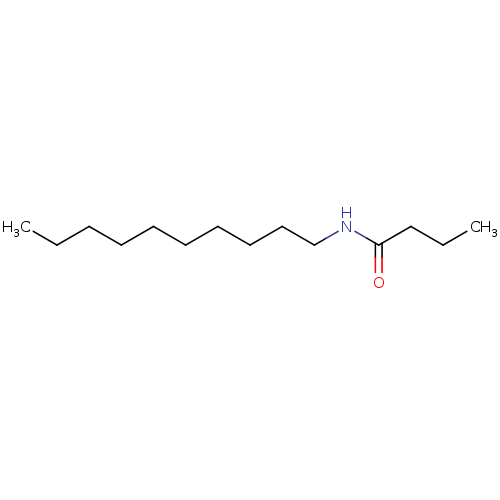
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
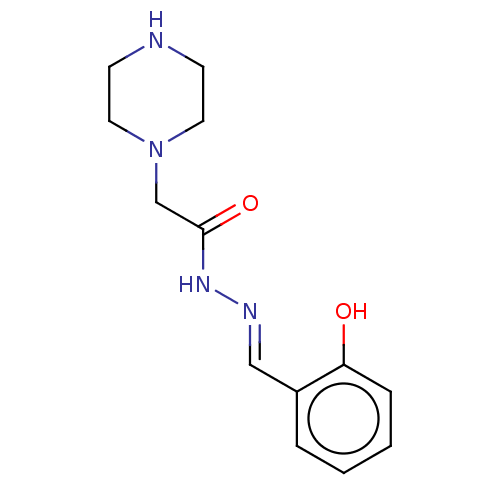
Affinity DataKi: 8.90E+3nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
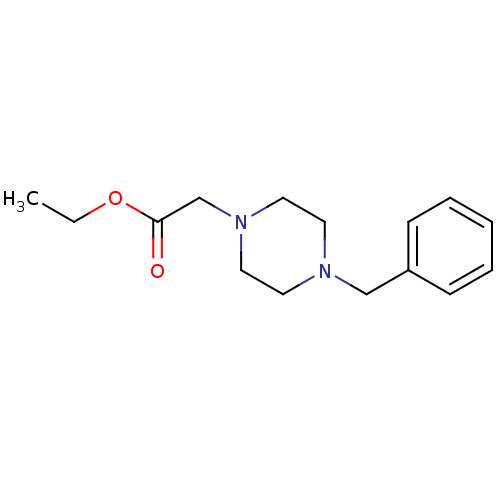
Affinity DataKi: 1.04E+4nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.57E+4nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 3.54E+4nMAssay Description:Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+5nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of human LDH-A using pyruvate as substrate and NADH as cofactor at 125 uM after 5 mins by calorimetric assay relative to controlMore data for this Ligand-Target Pair
Affinity DataEC50: 4.50E+3nMpH: 7.4 T: 2°CAssay Description:Procaspase-7 activation assay; activation of procaspase-7 to caspase-7. More data for this Ligand-Target Pair
Affinity DataEC50: 8.50E+3nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 5.00E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.50E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.50E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 5.00E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 6.60E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+4nMAssay Description:Activation of procaspase 2 after 24 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 220nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: 430nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Procaspase-3 was recombinantly expressed in E. coli with an N-terminal His-6 tag (SEQ ID NO: 29) and purified. Immunoblotting was performed with an a...More data for this Ligand-Target Pair
Affinity DataEC50: 220nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair
Affinity DataEC50: 430nMpH: 7.4 T: 2°CAssay Description:Procaspase-3 activation assay; activation of procaspase-3 to caspase-3. More data for this Ligand-Target Pair

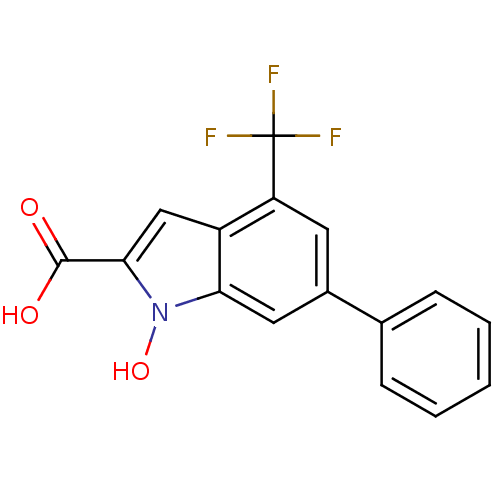
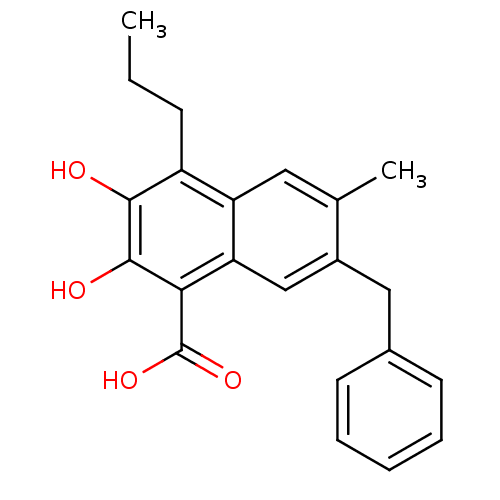
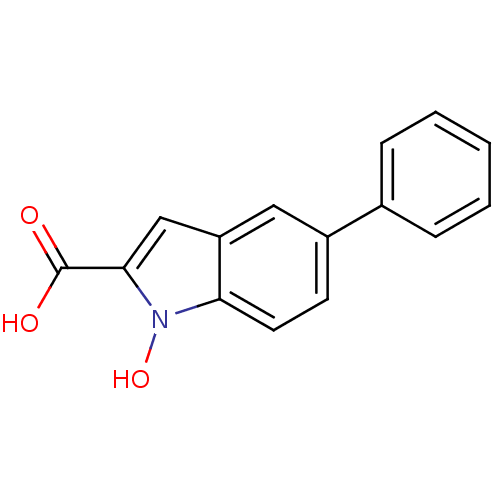
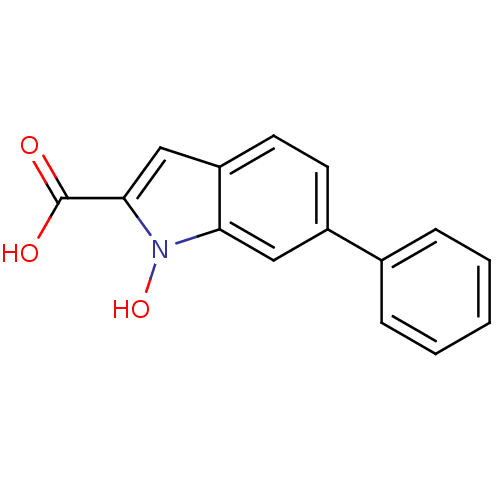

 3D Structure (crystal)
3D Structure (crystal)