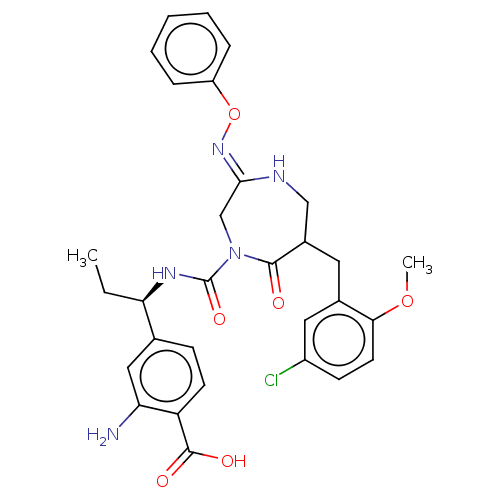
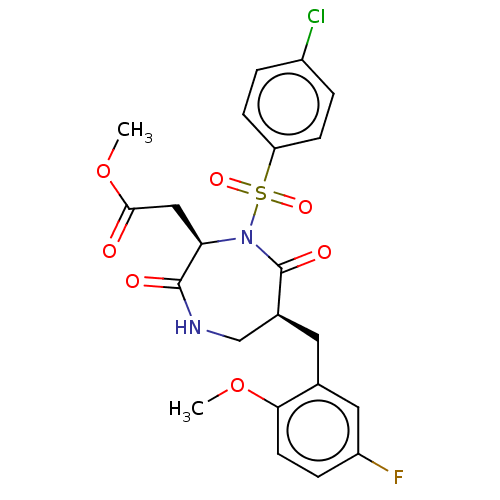
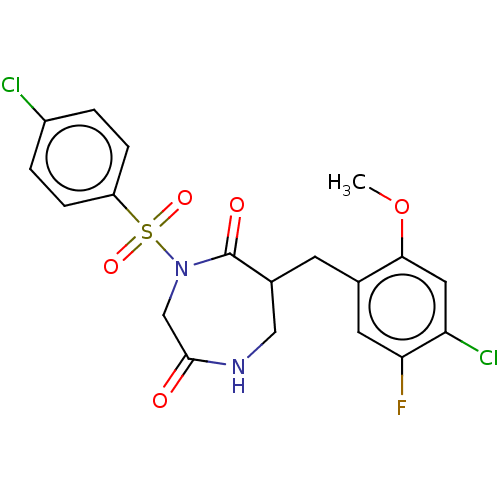
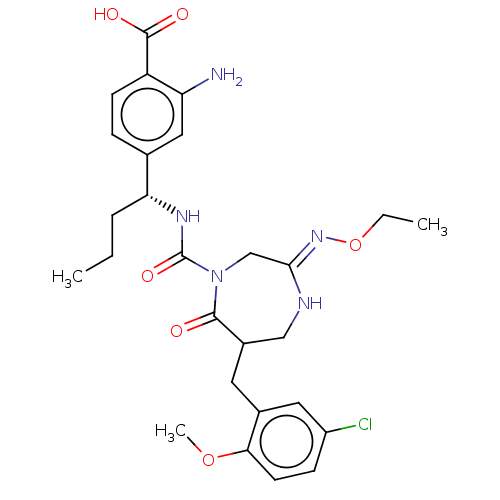
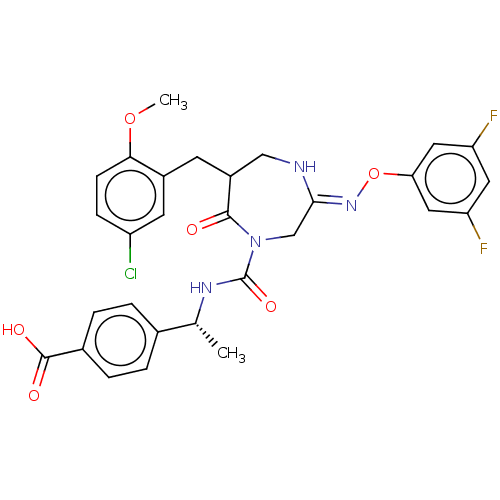
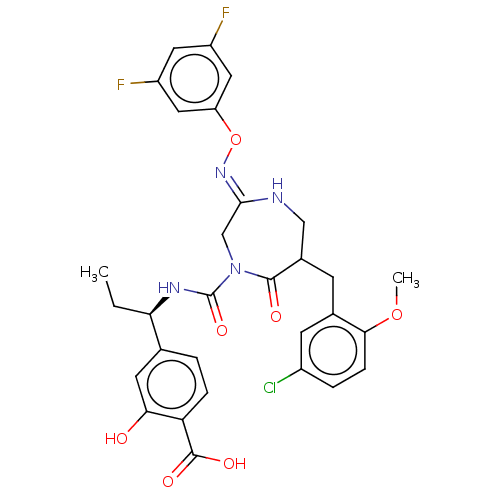
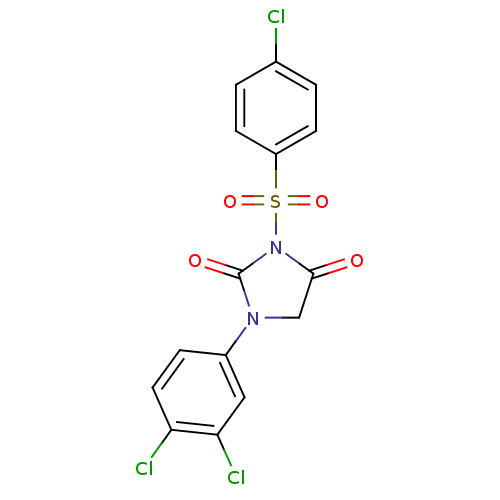
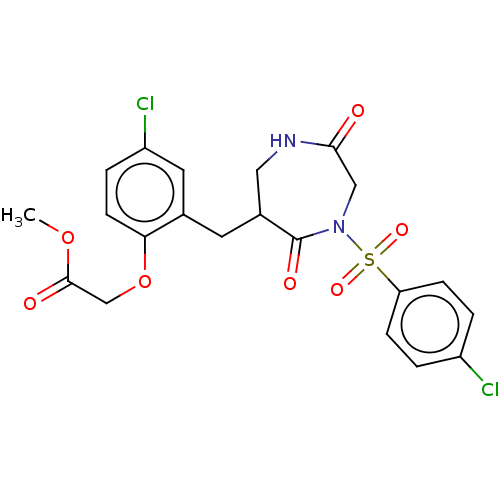
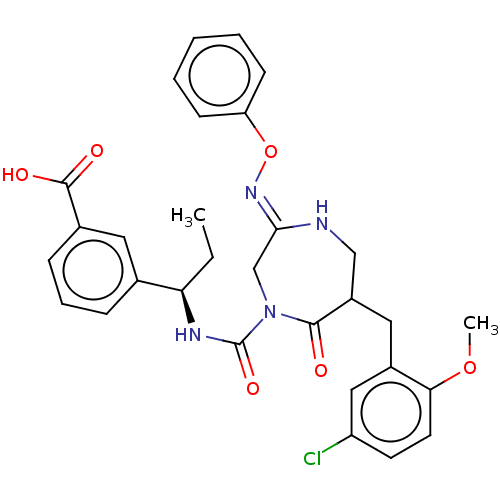
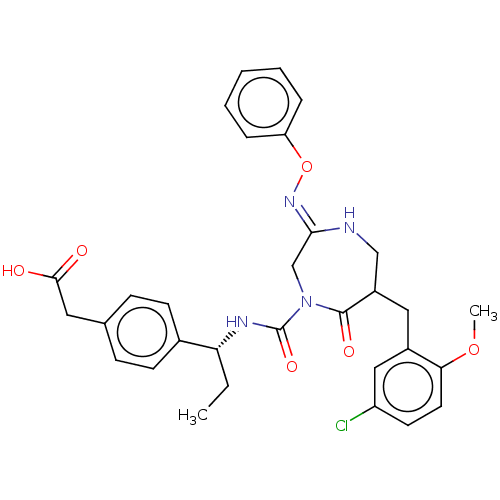
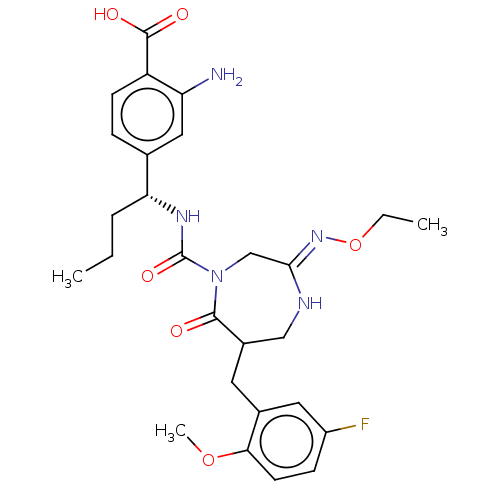
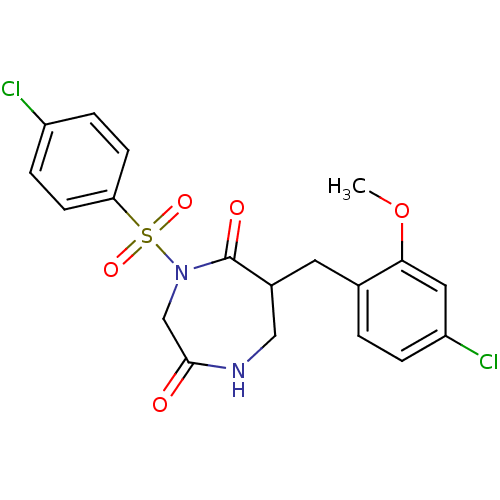
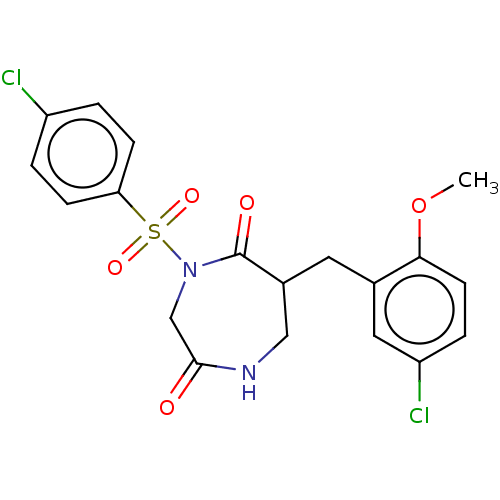
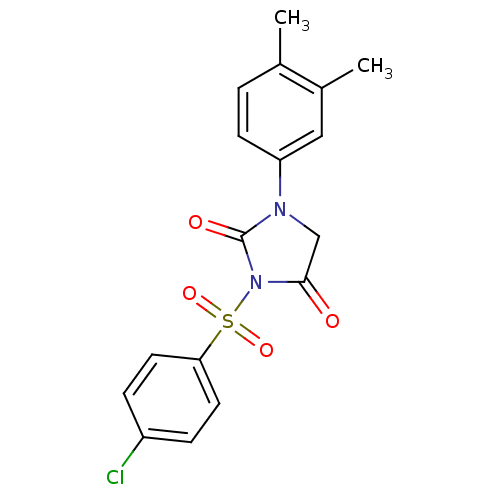
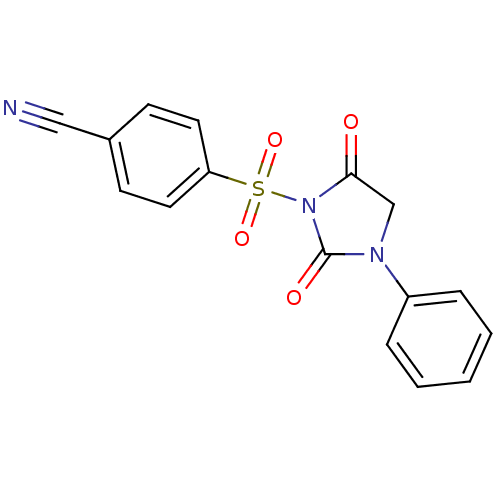
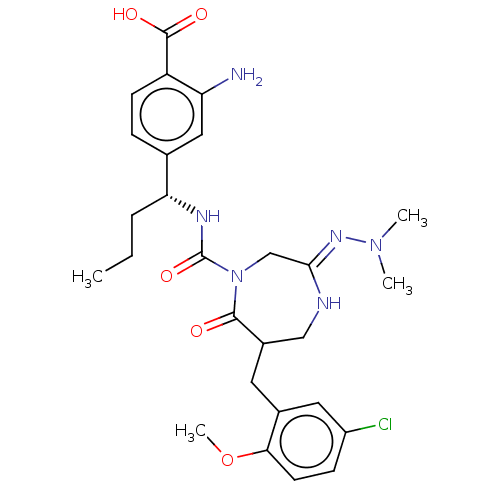
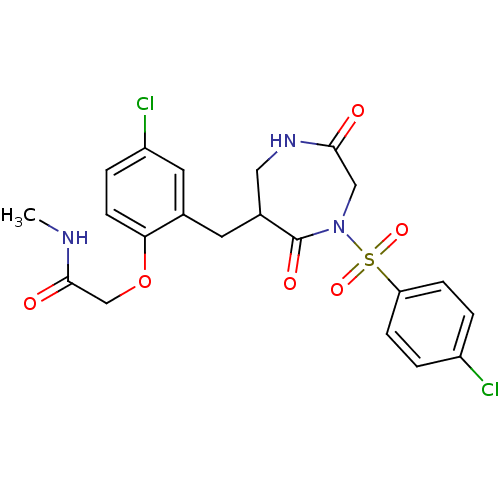
Affinity DataIC50: 3nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
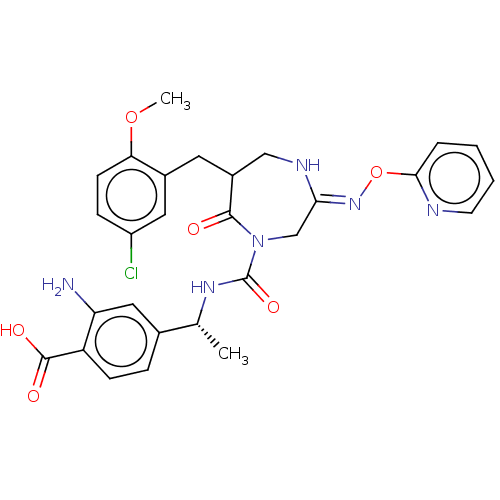
Affinity DataIC50: 4nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
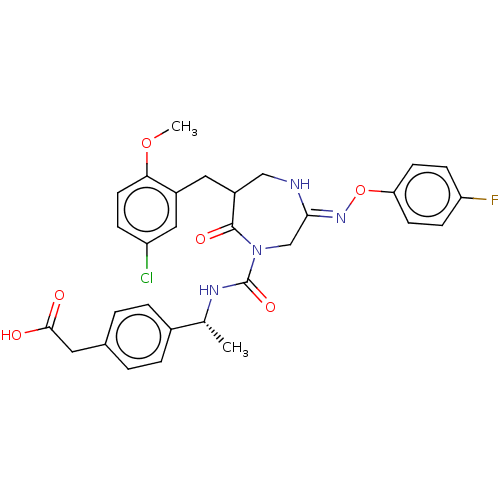
Affinity DataIC50: 7nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Inhibition of recombinant human chymase pre-incubated for 10 mins before Suc-Ala-Ala-Pro-Phe-MCA substrate addition and measured after 10 mins by flu...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibitory activity of the compounds for recombinant human chymase was measured by method of Pasztor et al. (Pasztor et al., Acta Biol. Hung. 42:285-...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibitory activity against human heart chymase in vitro.More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 7.5 T: 2°CAssay Description:The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of human recombinant chymaseMore data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of recombinant human KLK7 using MOCAc-Arg-Pro-Lys-Pro-Val-Glu-Nva-Trp-Arg-Lys(Dnp)-NH2 as substrate preincubated for 10 mins followed by s...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitroMore data for this Ligand-Target Pair

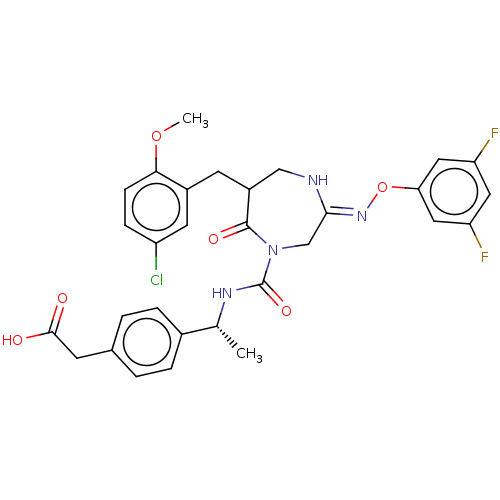
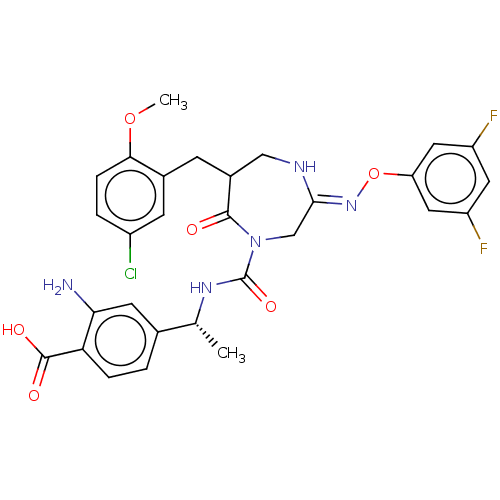
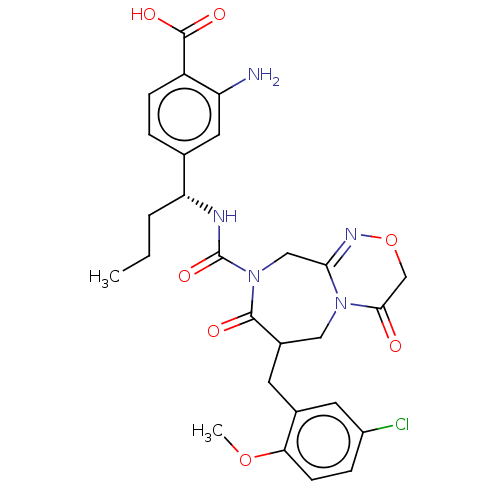
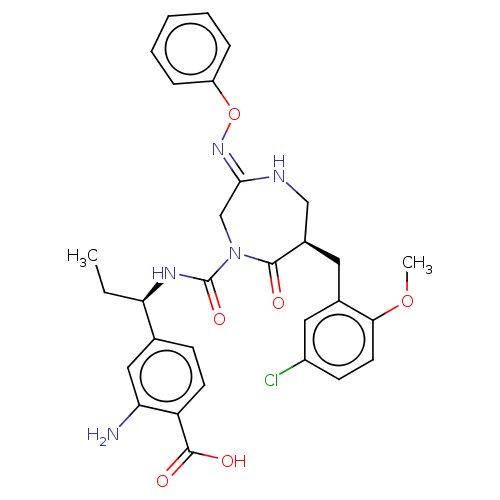
 3D Structure (crystal)
3D Structure (crystal)