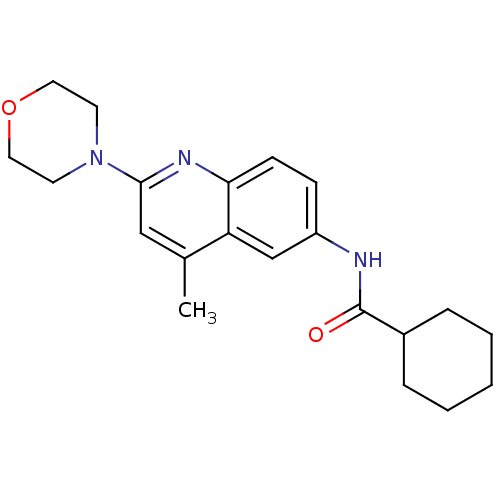
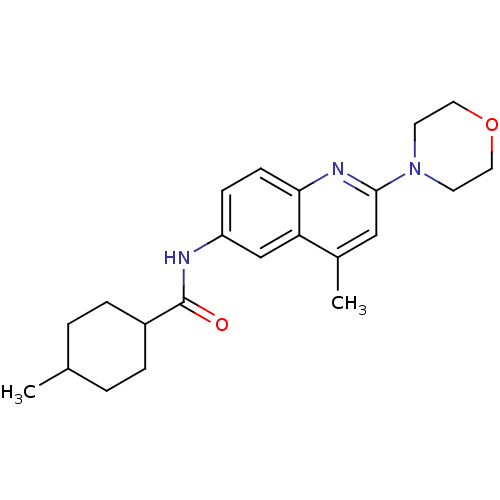
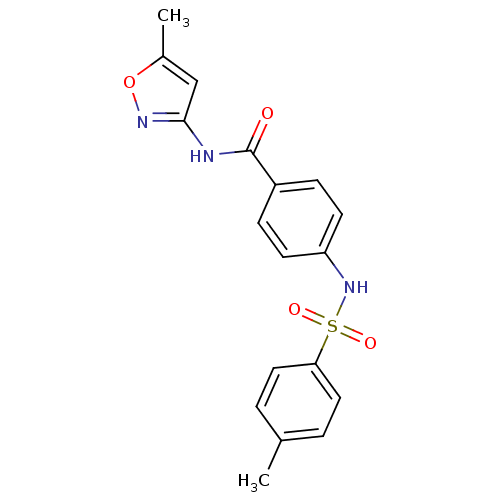
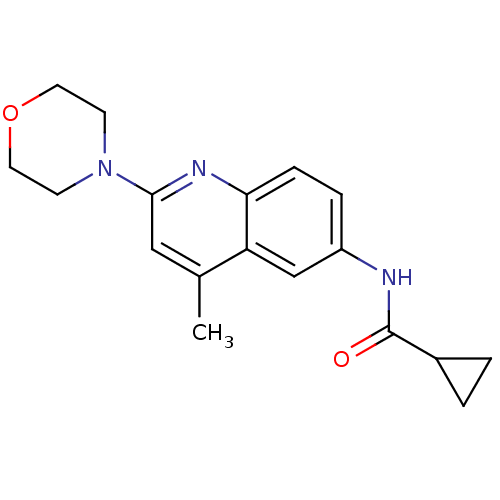
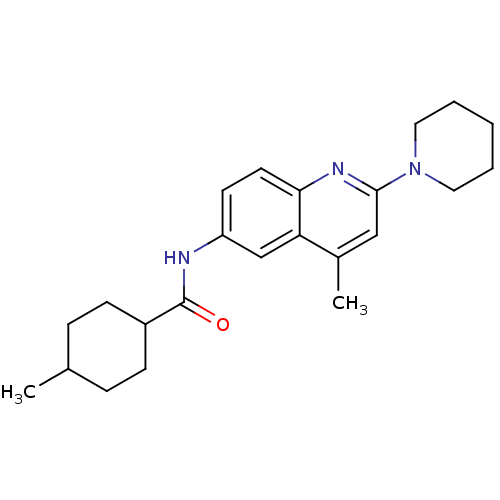
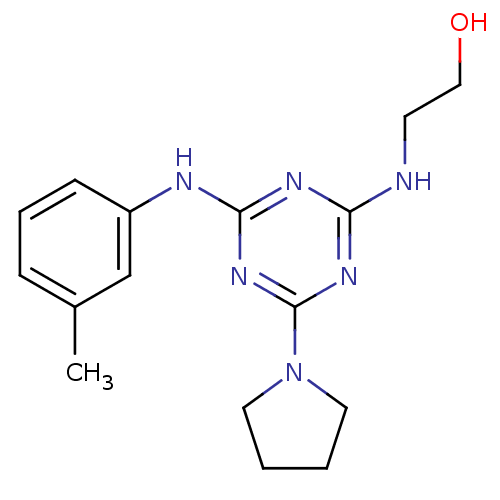
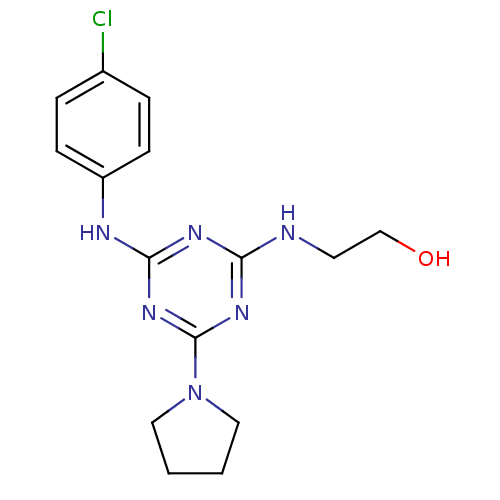
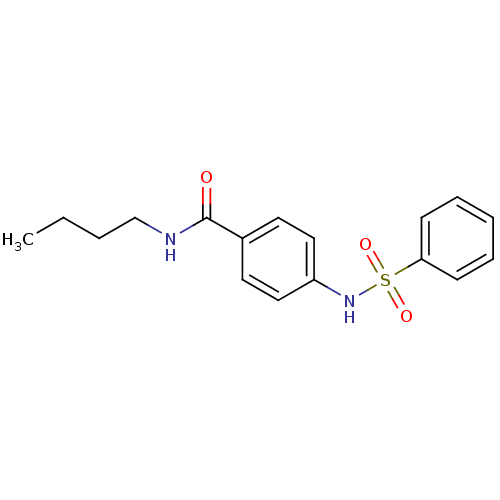
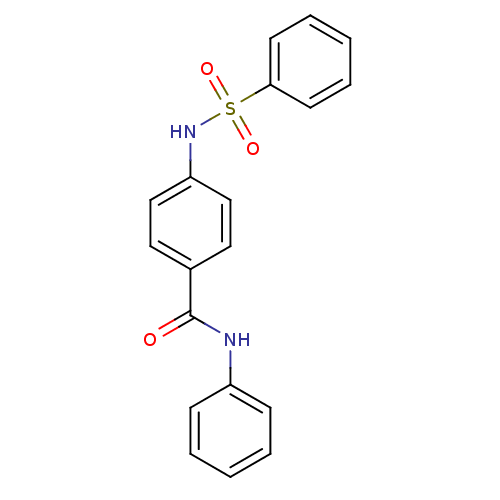
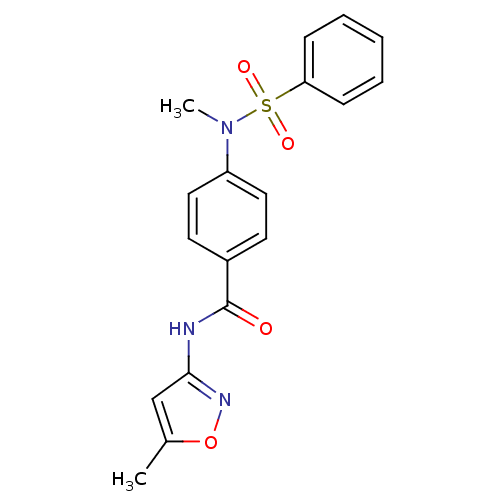
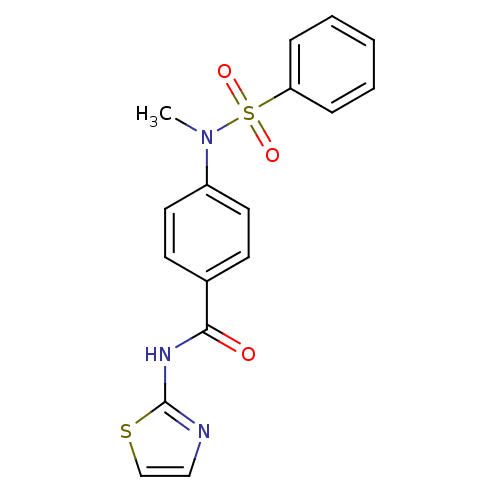
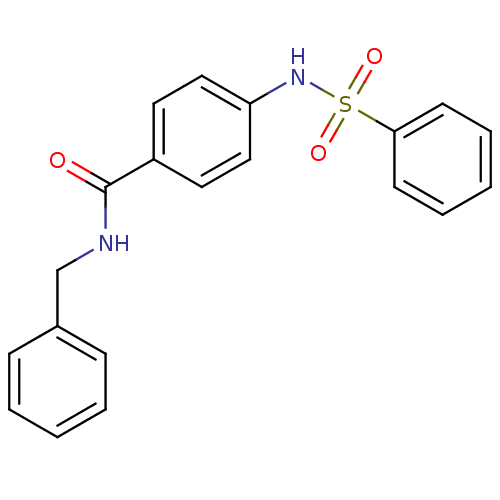
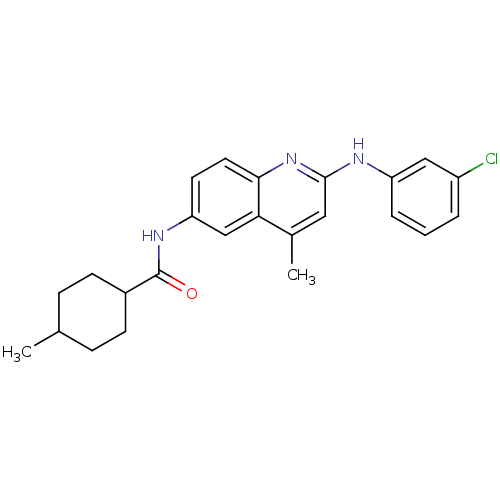
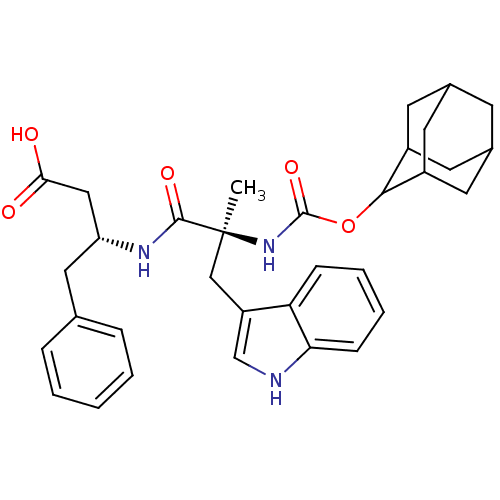
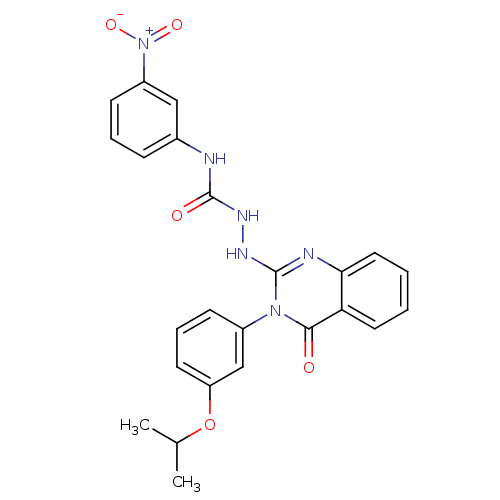
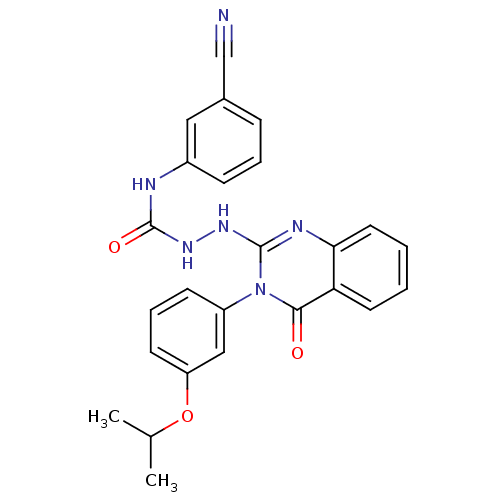
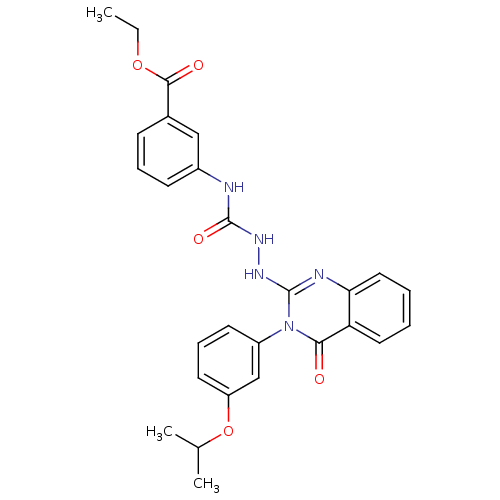
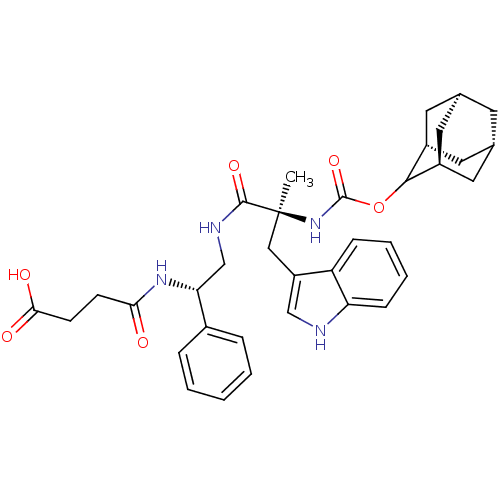
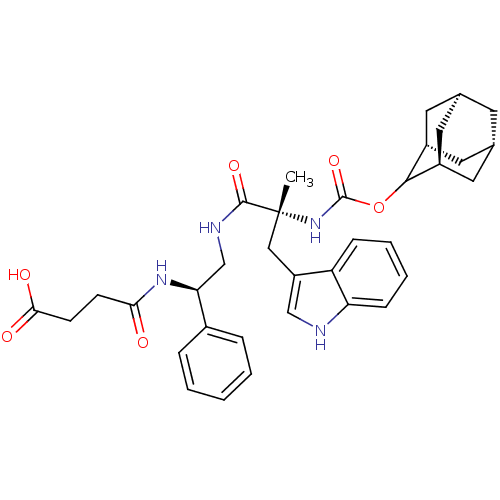
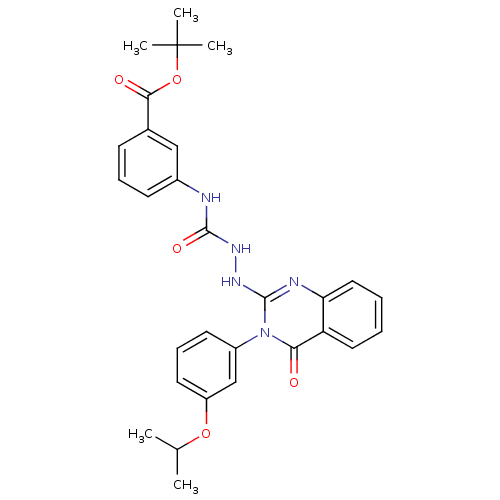
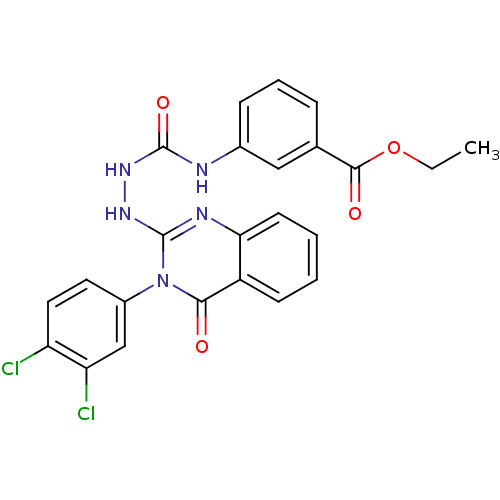
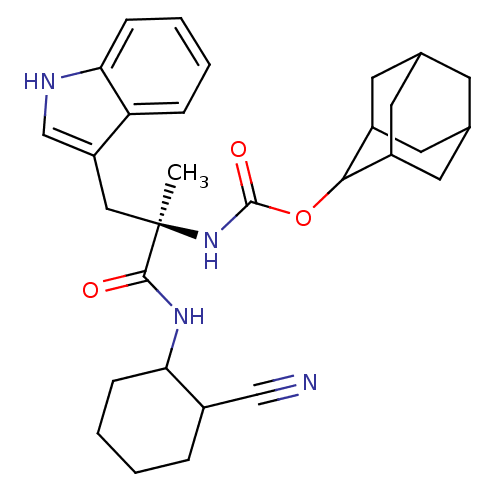
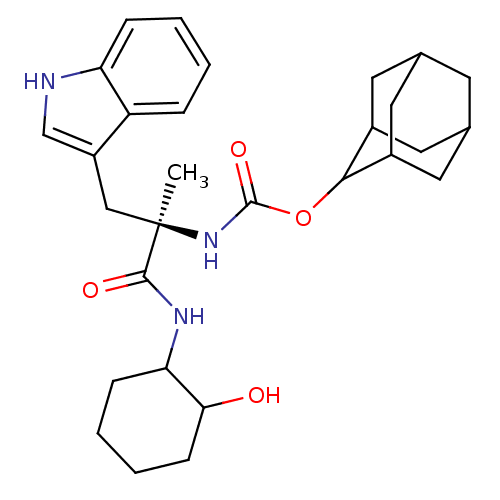
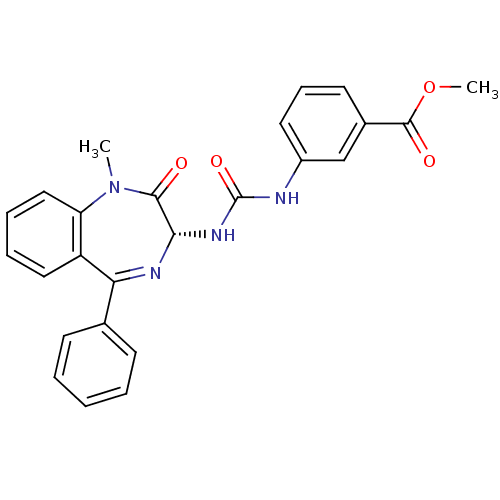
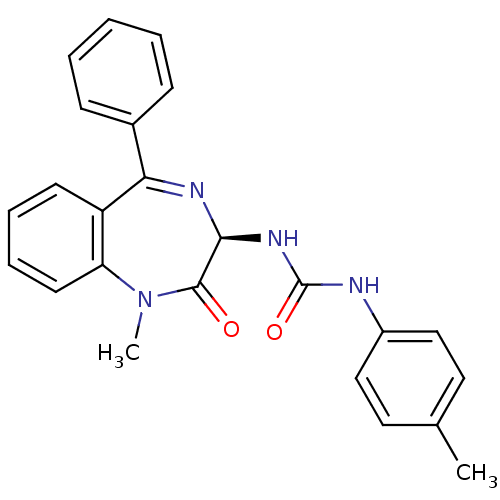
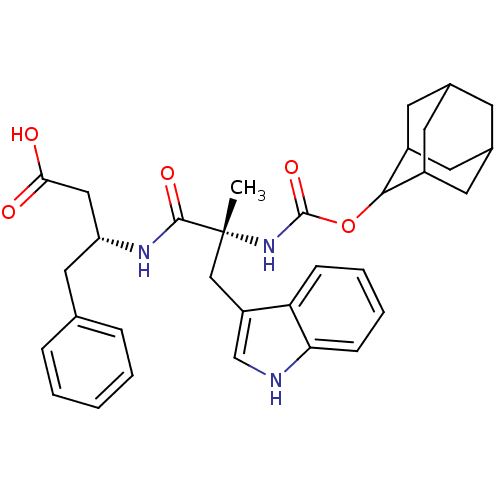
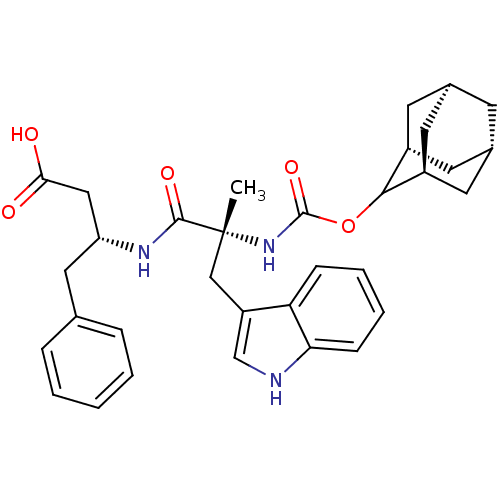
Affinity DataKi: 21nM ΔG°: -43.2kJ/mole IC50: 31nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
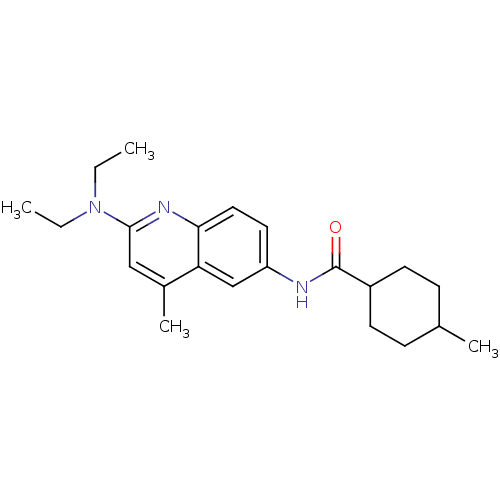
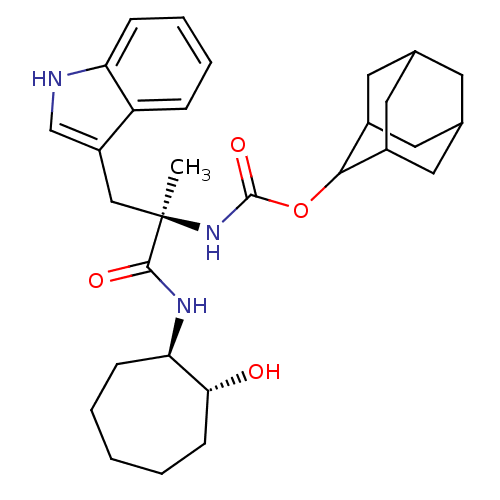
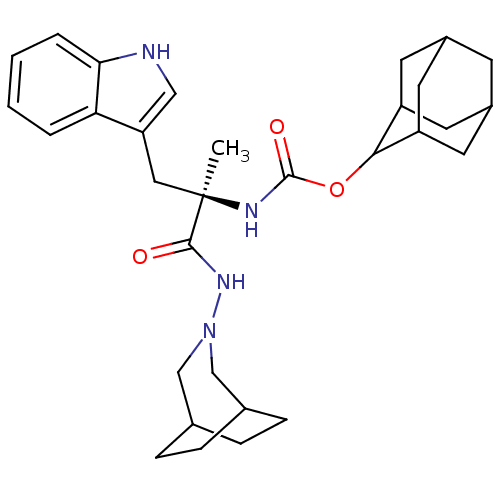
Affinity DataKi: 52nM ΔG°: -41.0kJ/mole IC50: 103nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
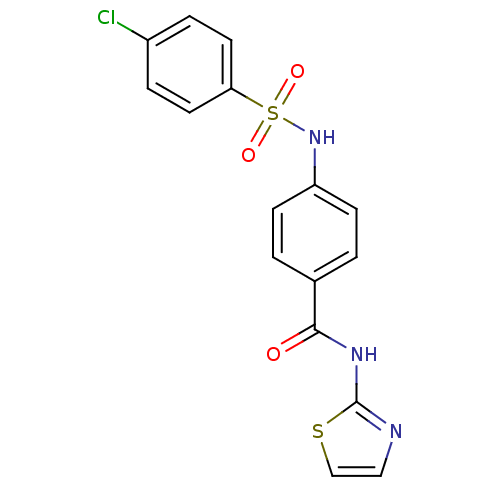
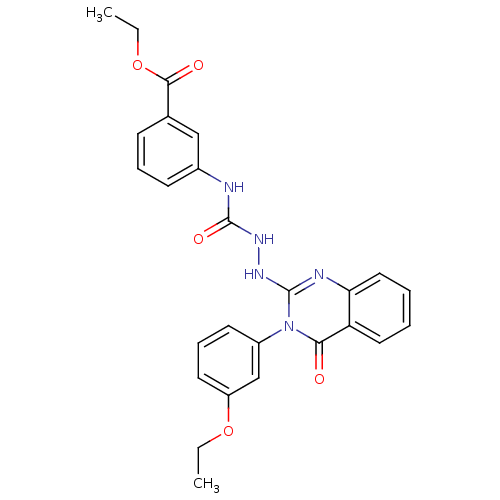
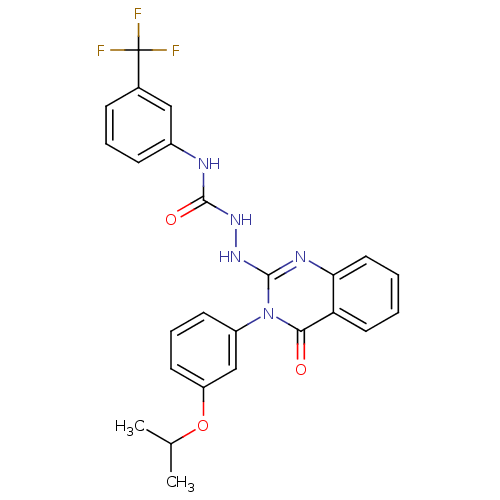
Affinity DataKi: 55nM ΔG°: -40.9kJ/mole IC50: 133nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
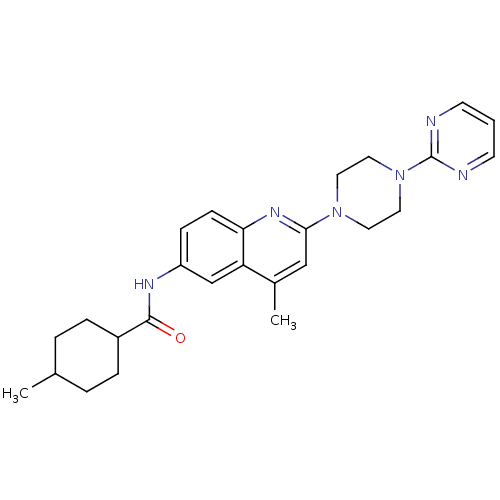
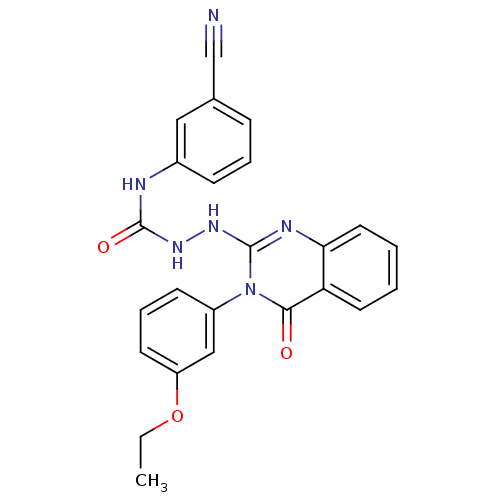
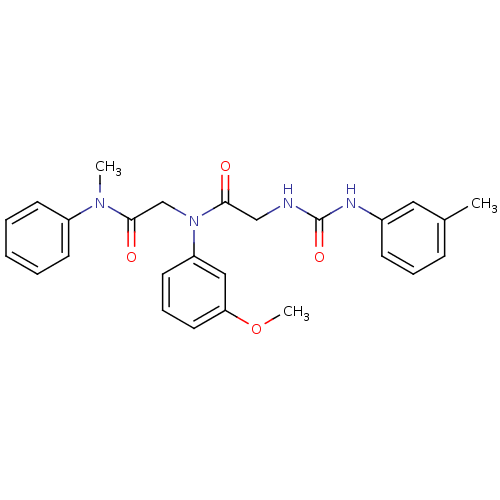
Affinity DataKi: 56nM ΔG°: -40.8kJ/mole IC50: 63nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 102nM ΔG°: -39.4kJ/mole IC50: 168nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -39.0kJ/mole IC50: 268nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 121nM ΔG°: -39.0kJ/mole IC50: 183nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 184nM ΔG°: -37.9kJ/mole IC50: 452nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 320nM ΔG°: -36.6kJ/mole IC50: 430nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 514nM ΔG°: -35.4kJ/mole IC50: 1.06E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 556nM ΔG°: -35.2kJ/mole IC50: 1.29E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 975nM ΔG°: -33.8kJ/mole IC50: 2.45E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 2.78E+3nM ΔG°: -31.3kJ/mole IC50: 4.31E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 4.23E+3nM ΔG°: -30.3kJ/mole IC50: 7.73E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 7.15E+3nM ΔG°: -29.0kJ/mole IC50: 2.46E+4nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 8.44E+3nM ΔG°: -28.6kJ/mole IC50: 2.96E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 1.34E+4nM ΔG°: -27.4kJ/mole IC50: 6.46E+3nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 1.92E+4nM ΔG°: -26.6kJ/mole IC50: 2.52E+4nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 2.34E+4nM ΔG°: -26.1kJ/mole IC50: 3.44E+4nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 5.06E+4nM ΔG°: -24.2kJ/mole IC50: >1.00E+5nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 1.22E+5nM ΔG°: -22.0kJ/molepH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:In vitro inhibition of [125I]- Bolton Hunter CCK-8 binding to Cholecystokinin type B receptor in the mouse cerebral cortex.More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 0.990nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In vitro inhibition of [125I]- Bolton Hunter CCK-8 binding to Cholecystokinin type B receptor in the mouse cerebral cortex.More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:In vitro inhibition of [125I]- Bolton Hunter CCK-8 binding to Cholecystokinin type B receptor in the mouse cerebral cortex.More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of [125 I]CCK-8 binding to Cholecystokinin type B receptor of mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor in mouse cerebral cortex using [125I]-Bolton Hunter CCK-8 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:In vitro inhibition of [125I]- Bolton Hunter CCK-8 binding to Cholecystokinin type B receptor in the mouse cerebral cortex.More data for this Ligand-Target Pair