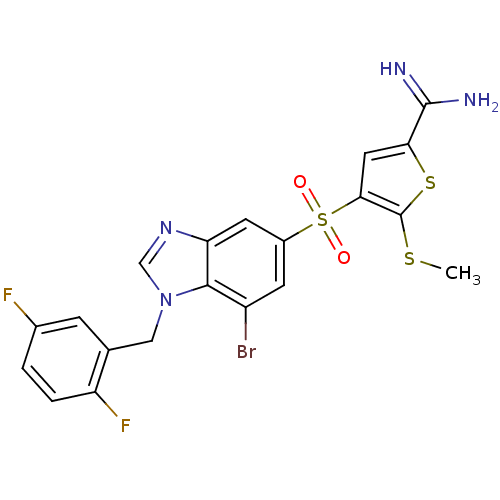
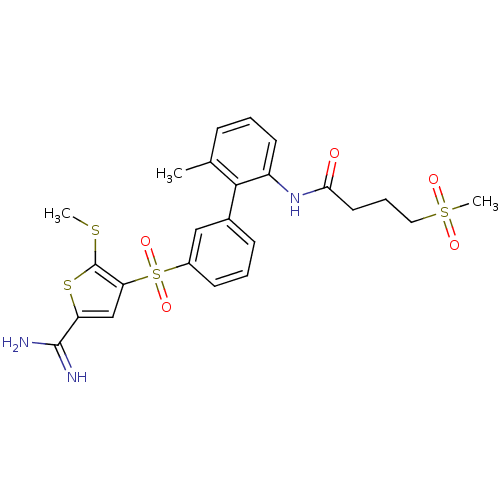
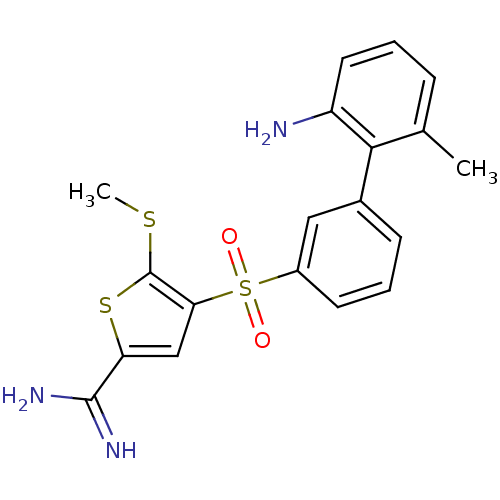
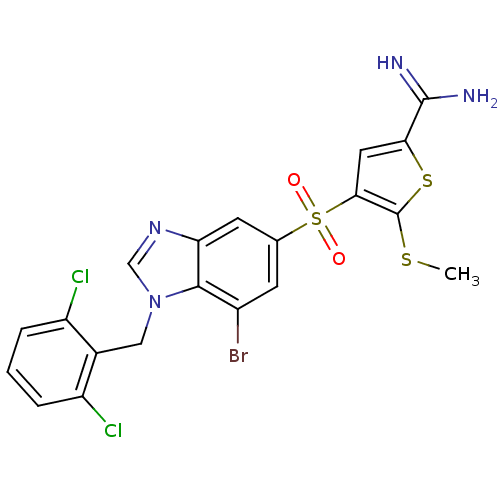
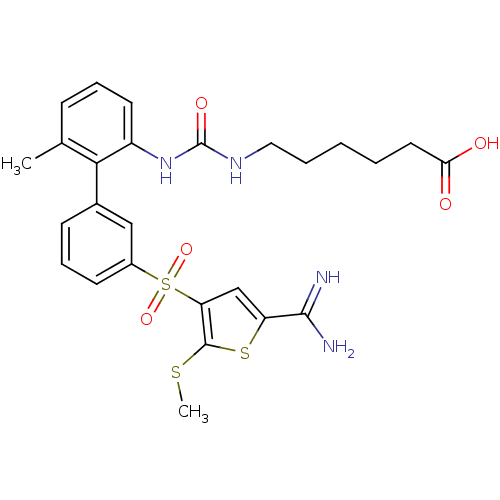
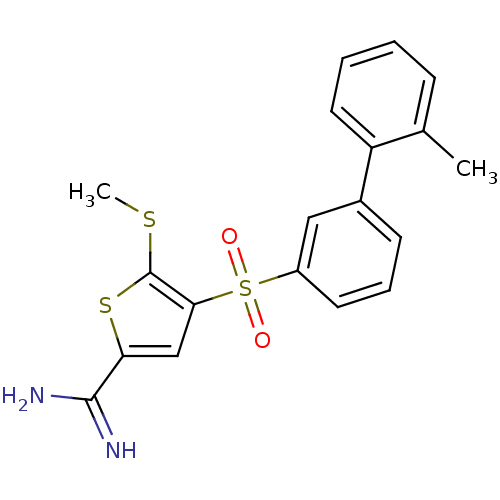
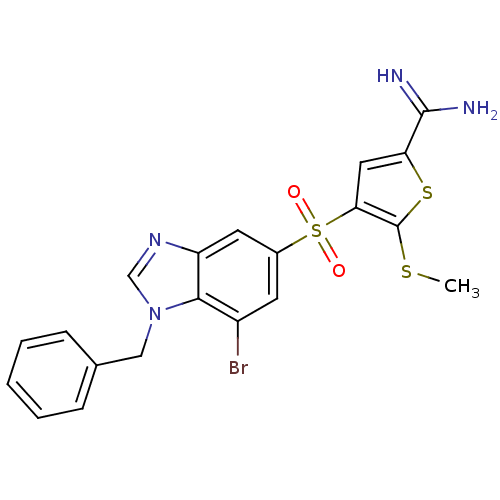
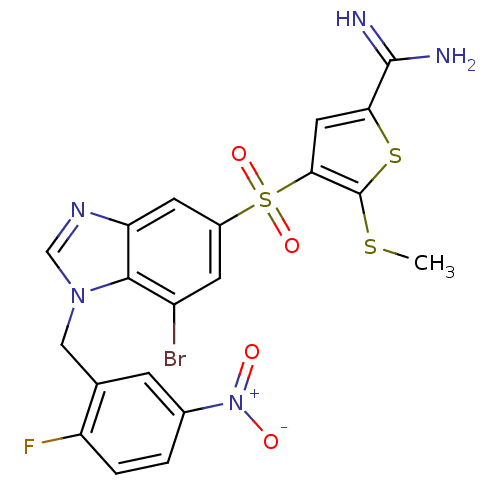
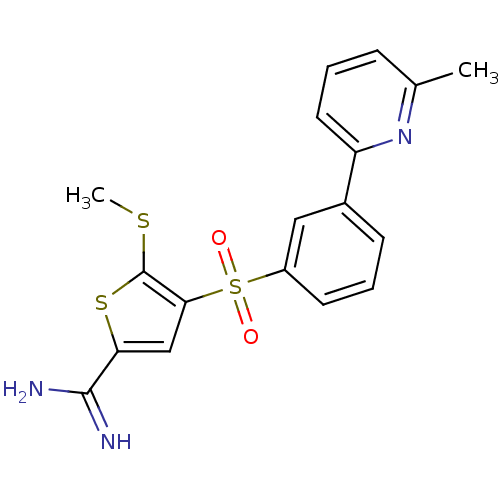
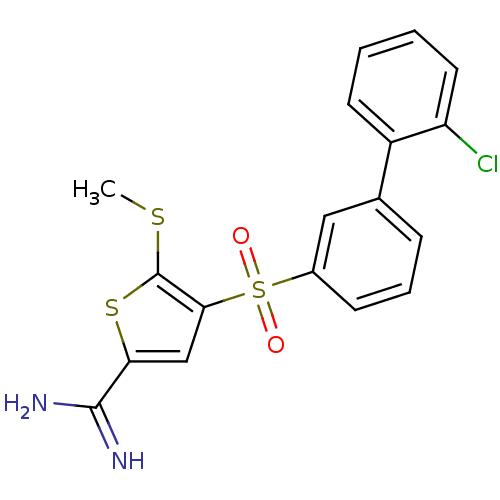
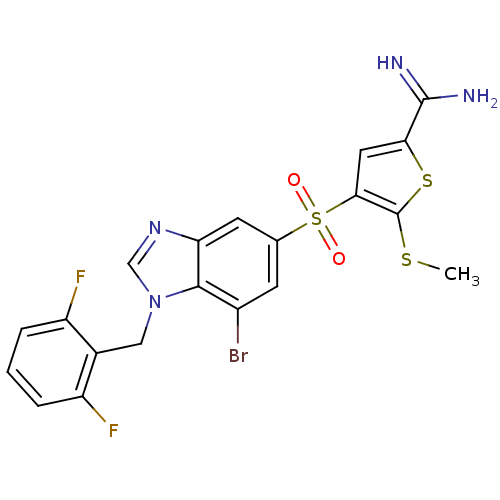
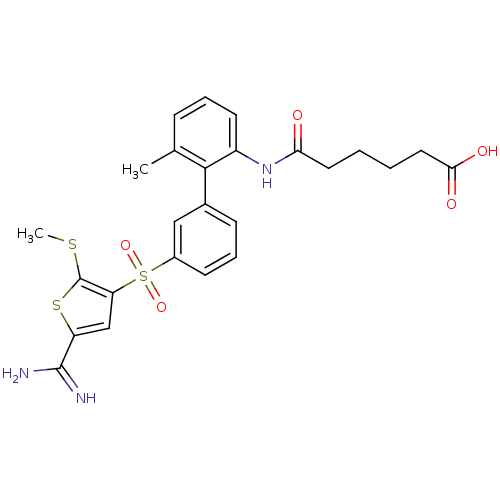
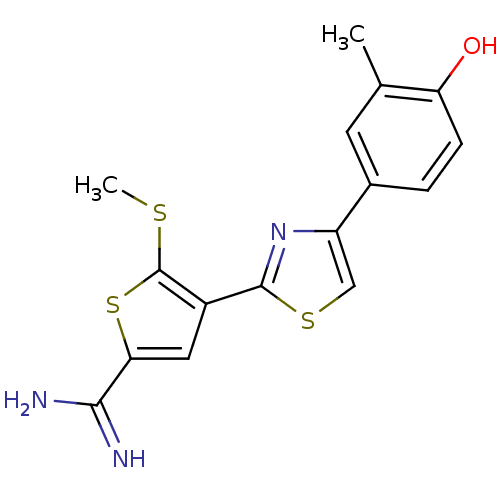
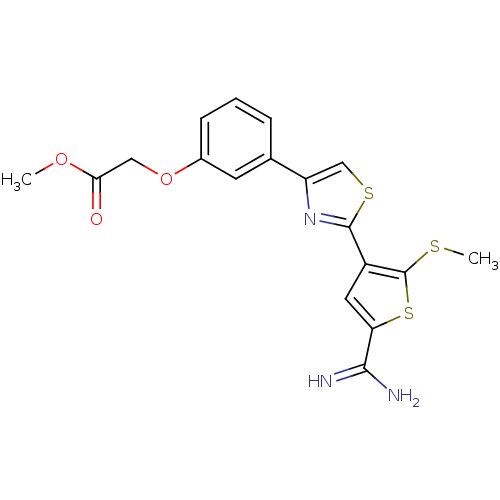
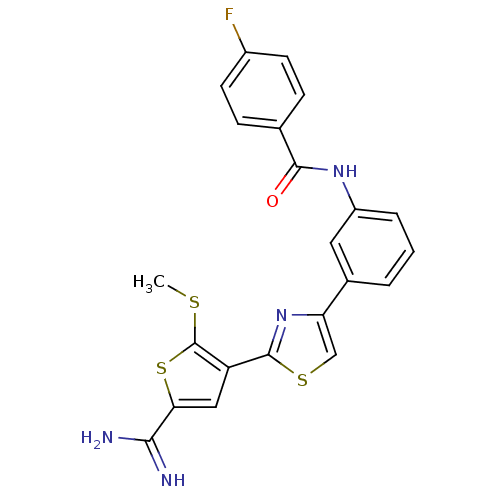
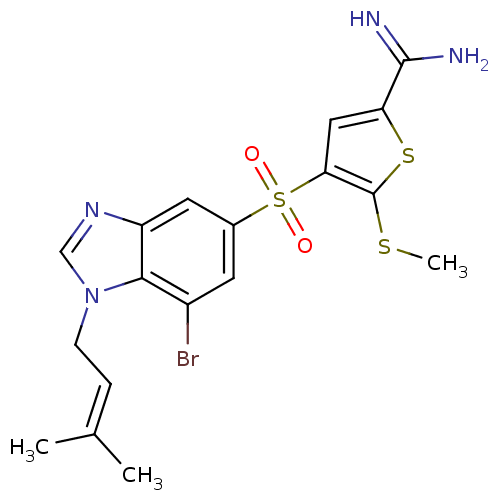
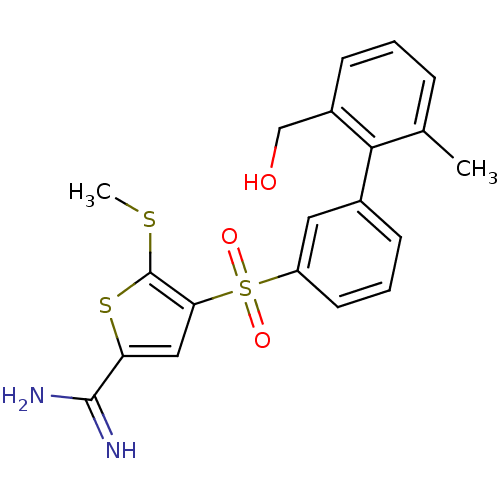
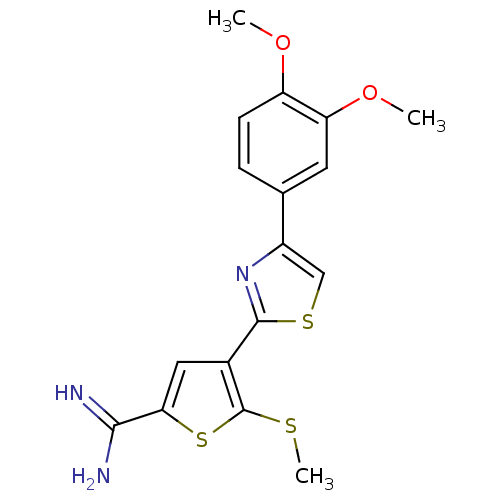
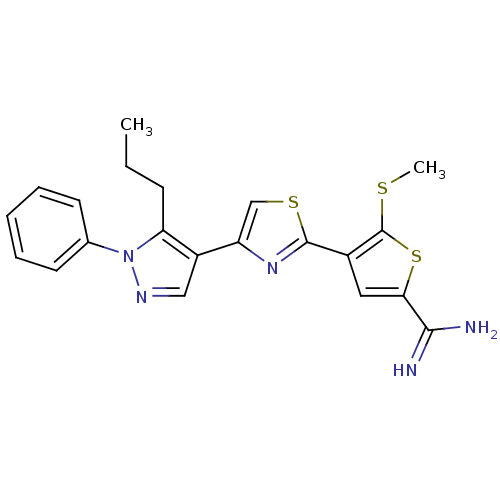
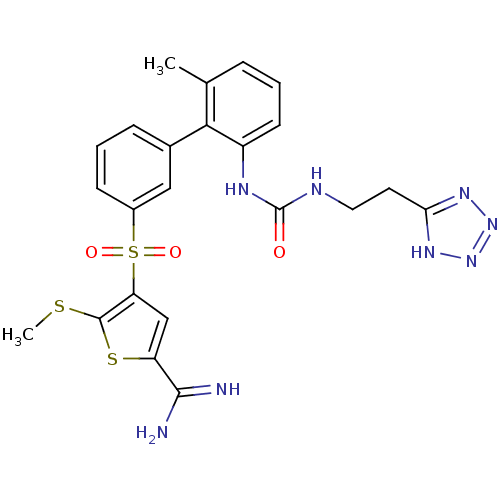
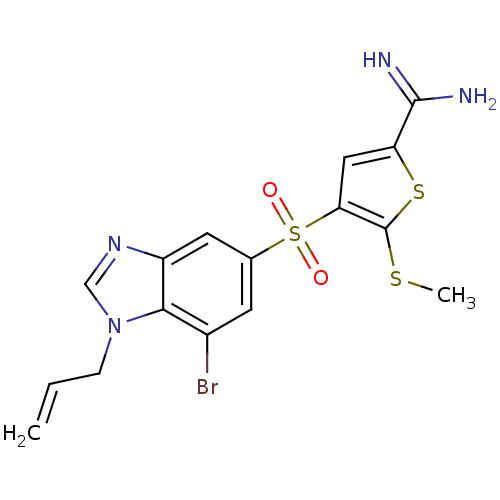
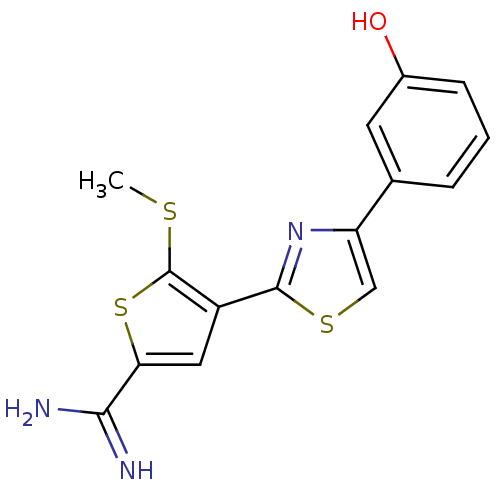
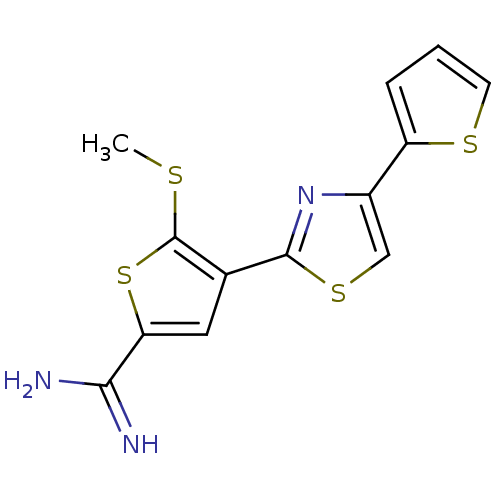
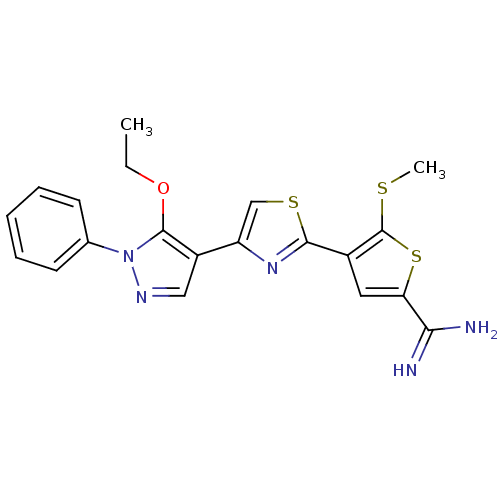
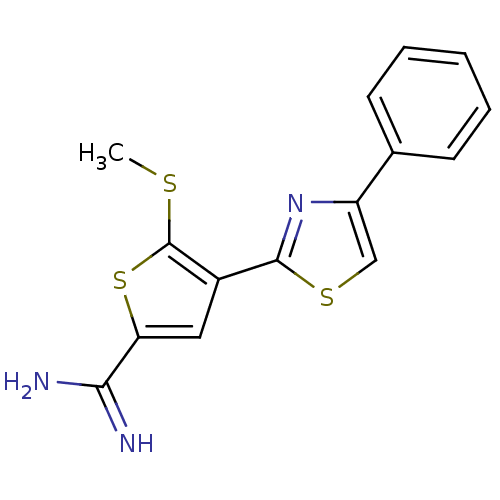
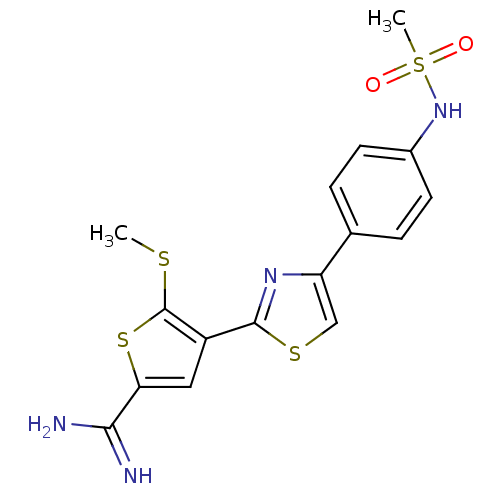
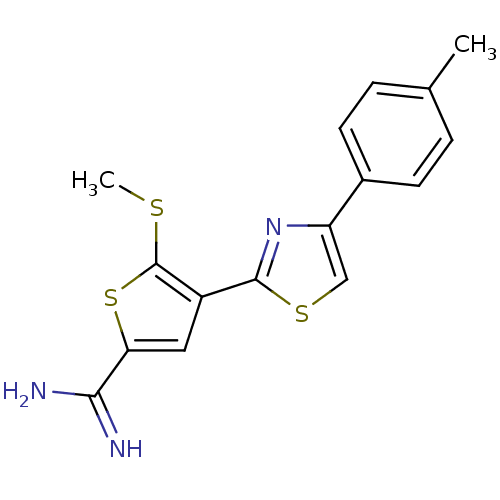
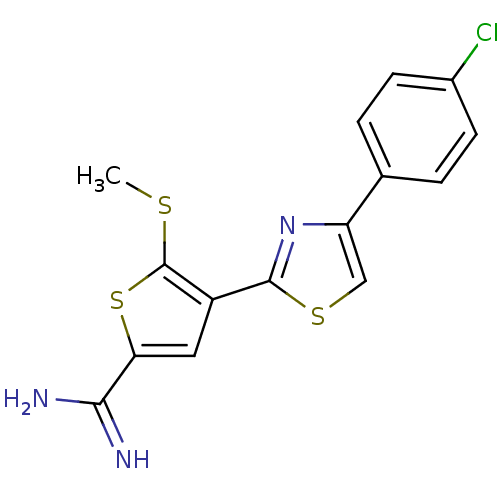
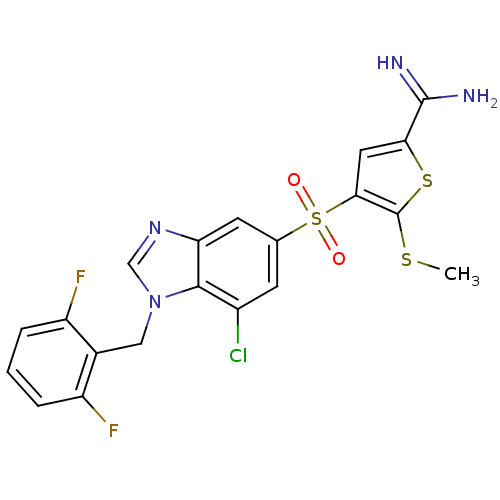
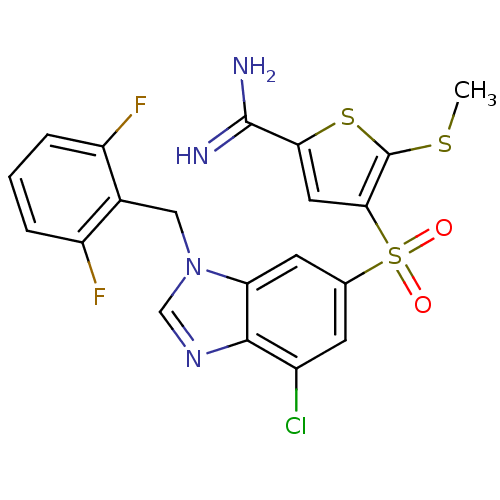
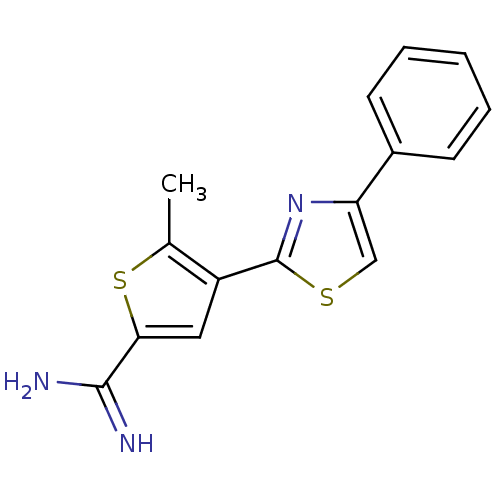
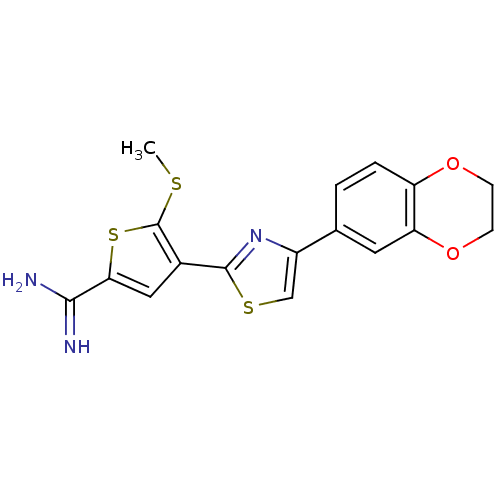
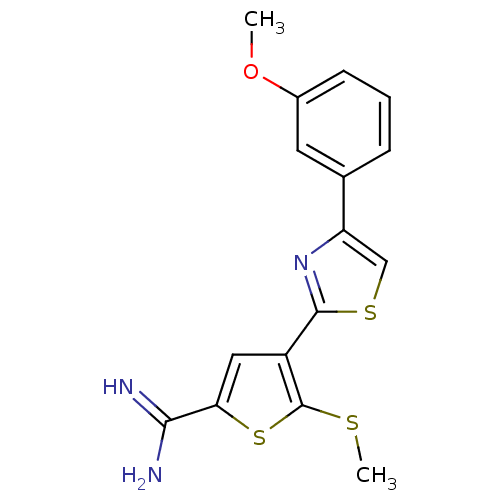
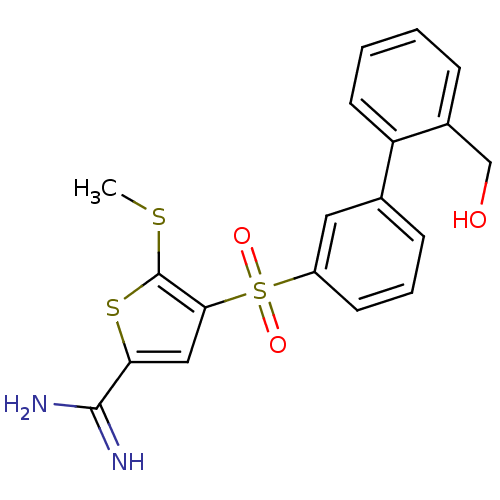
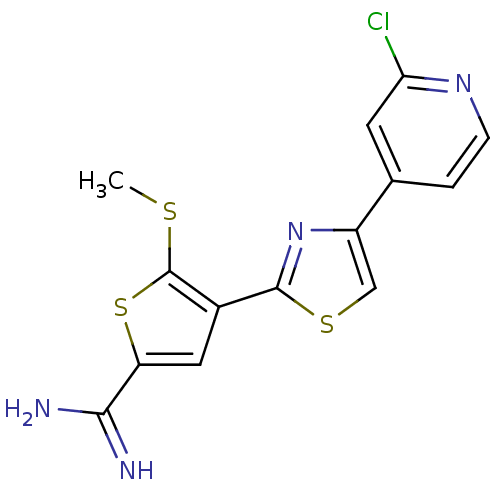
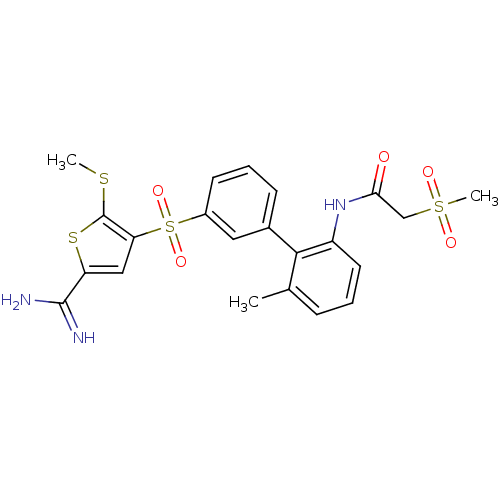
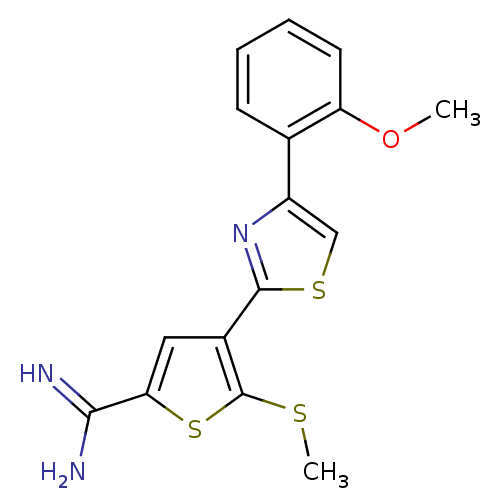
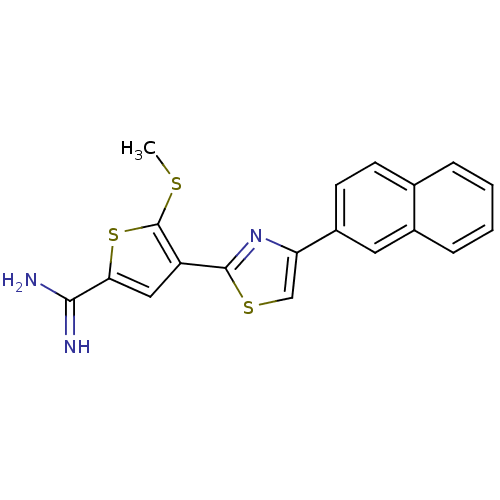
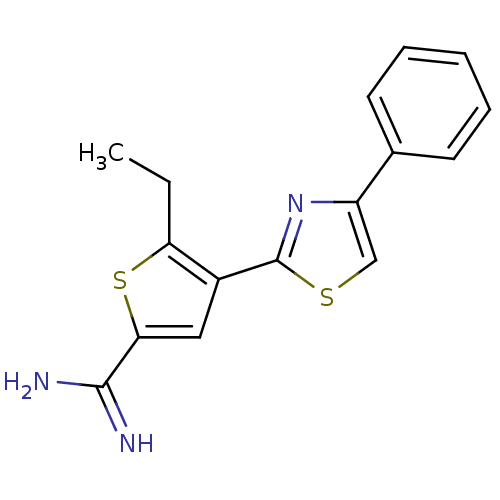
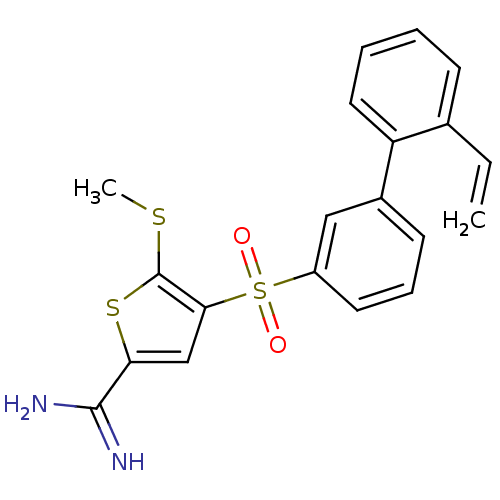
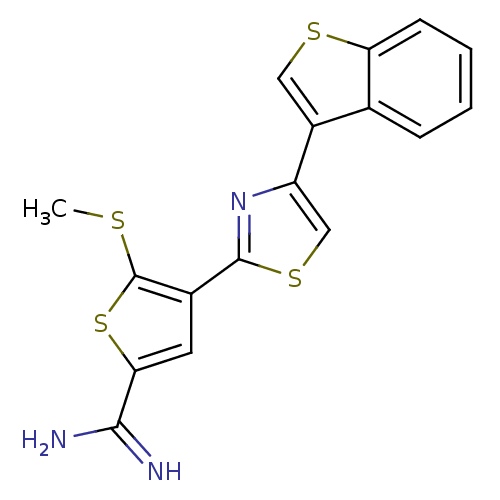
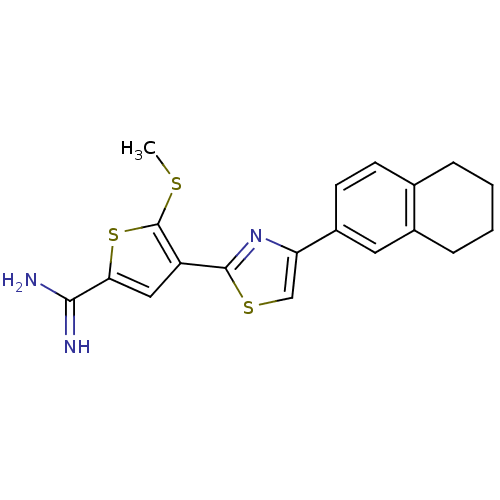
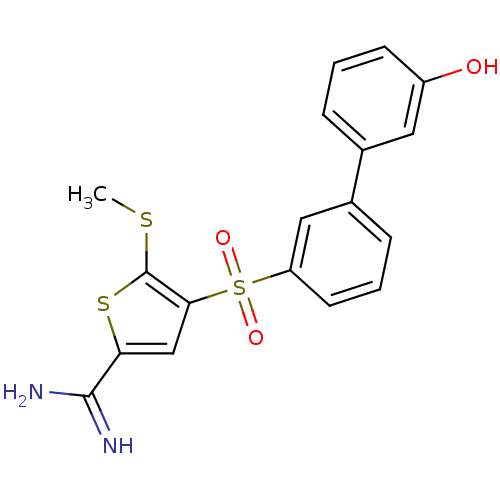
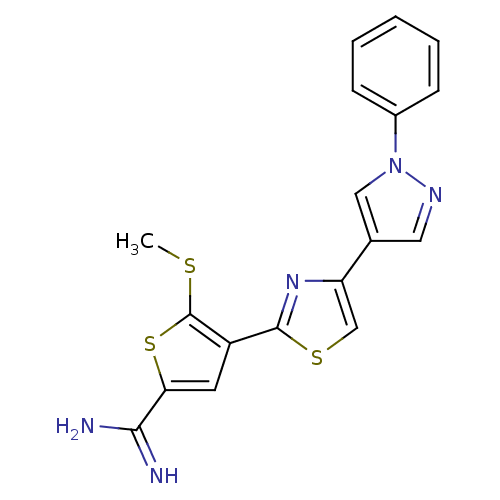
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 42nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 44nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 44nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 58nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 64nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 86nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 91nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 99nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 102nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 108nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 115nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 138nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 138nMAssay Description:Inhibition of Human kidney cell urokinaseMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 141nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
3-Dimensional Pharmaceuticals
Curated by ChEMBL
3-Dimensional Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 145nMAssay Description:Inhibitory activity against serine protease urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair