

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM14058 CHEMBL316053::N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxo-pyrrolidin-3-yl]-7-thia-2-azabicyclo[4.3.0]nona-2,4,8,10-tetraene-8-sulfonamide::N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxopyrrolidin-3-yl]thieno[3,2-b]pyridine-2-sulfonamide::RPR208815
SMILES: Nc1nccc2ccc(CN3CC[C@H](NS(=O)(=O)c4cc5ncccc5s4)C3=O)cc12
InChI Key: InChIKey=NVKDOURNRJCKJE-UHFFFAOYSA-N
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Human) | BDBM14058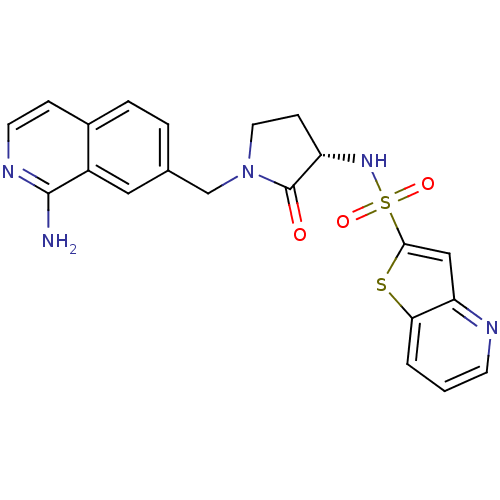 (N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxop...) | GoogleScholar | UniChem | 22 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bovine) | BDBM14058 (N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxop...) | GoogleScholar | UniChem | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Human) | BDBM14058 (N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxop...) | GoogleScholar | UniChem | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bovine) | BDBM14058 (N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxop...) | GoogleScholar | UniChem | >2.90E+3 | >-7.47 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Human) | BDBM14058 (N-[(3S)-1-[(1-aminoisoquinolin-7-yl)methyl]-2-oxop...) | GoogleScholar | UniChem | >4.00E+3 | >-7.28 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||