

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM341808 US10544104, Compound 124::US9765037, Compound 124
SMILES: CC(C)COc1ccc2cc(ccc2c1)-c1nn(CC2CCN(C)CC2)c2ncnc(N)c12
InChI Key: InChIKey=GOKRPYKIPRDTFC-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Human) | BDBM341808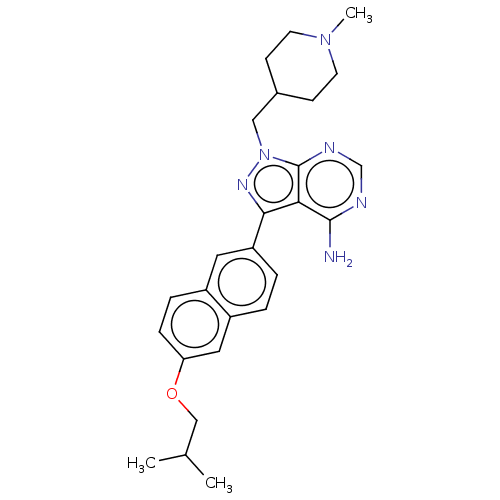 (US9765037, Compound 124 | US10544104, Compound 124) | GoogleScholar | UniChem | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Human) | BDBM341808 (US9765037, Compound 124 | US10544104, Compound 124) | GoogleScholar | UniChem | n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Human) | BDBM341808 (US9765037, Compound 124 | US10544104, Compound 124) | GoogleScholar | UniChem | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Human) | BDBM341808 (US9765037, Compound 124 | US10544104, Compound 124) | GoogleScholar | UniChem | n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||