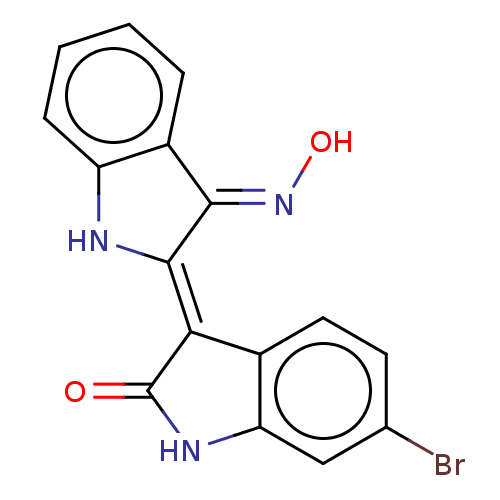
BDBM50012188 6BIO
SMILES O\N=C1\C(\Nc2ccccc\12)=C1\C(=O)Nc2cc(Br)ccc12
InChI Key InChIKey=DDLZLOKCJHBUHD-UHFFFAOYSA-N
Data 25 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 25 hits for monomerid = 50012188
Found 25 hits for monomerid = 50012188
TargetGlycogen synthase kinase-3 beta/[Tau protein] kinase(Pig)
Zunyi Medical University
Curated by ChEMBL
Zunyi Medical University
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of porcine brain GSK-3alpha/beta incubated for 30 mins in presence of [33p]-gamma ATP by scintillation counter analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of His-tagged CDK2/cyclin E (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate in presence of [gam...More data for this Ligand-Target Pair
TargetNon-receptor tyrosine-protein kinase TYK2(Human)
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of TYK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibition of GST-tagged CDK5/p25 (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate as substrate in presence...More data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Human)
Institut De Chimie Des Substances Naturelles
Curated by ChEMBL
Institut De Chimie Des Substances Naturelles
Curated by ChEMBL
Affinity DataIC50: 52nMAssay Description:Inhibition of DYRK1A (unknown origin) assessed as inhibition of phosphate incorporation into substrate in presence of radiolabeled ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Inhibition of GST-tagged CDK2/cyclin A2 (unknown origin) expressed in Escherichia coli using histone H1 as substrate in presence of [gamma-33P]-ATP b...More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibition of GST-fused CDK5/p25 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Inhibition of CDK5 (unknown origin) assessed as inhibition of phosphate incorporation into substrate in presence of radiolabeled ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of His-tagged CDK1/cyclin B1 (unknown origin) expressed in Baculovirus infected Sf9 cells using histone H1 as substrate in presence of [ga...More data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Inhibition of CDK1 (unknown origin) assessed as inhibition of phosphate incorporation into substrate in presence of radiolabeled ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Inhibition of GST-tagged CDK9/CyclinT1 (unknown origin) expressed in Baculovirus infected Sf9 cells using YSPTSPS-2 KK peptide as substrate as substr...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
Friedrich-Schiller-University
Curated by ChEMBL
Friedrich-Schiller-University
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of 5-LO in fMLP-stimulated human monocytes assessed as reduction in HETE formationMore data for this Ligand-Target Pair
Affinity DataIC50: 527nMAssay Description:Inhibition of GST-tagged CDK4/cyclin D1 (unknown origin) expressed in Baculovirus infected Sf9 cells using RPPTLSPIPHIPR peptide as substrate in pres...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant aurora A kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant aurora C kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of human recombinant aurora B kinaseMore data for this Ligand-Target Pair
Target[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial(Human)
Zunyi Medical University
Curated by ChEMBL
Zunyi Medical University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human recombinant GST-fused PDK1 expressed in Sf9 cells incubated for 30 mins in presence of [gamma-33P]ATP by scintillation counter an...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of GSK3beta (unknown origin)More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
Friedrich-Schiller-University
Curated by ChEMBL
Friedrich-Schiller-University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 20 uM A23187/AA ionopho...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
Friedrich-Schiller-University
Curated by ChEMBL
Friedrich-Schiller-University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of 5-LO in human neutrophils assessed as product formation by cell-intact assay relative to control in presence of 2.5 uM A23187 ionophoreMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
Friedrich-Schiller-University
Curated by ChEMBL
Friedrich-Schiller-University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of 5-LO in LPS-stimulated human neutrophils assessed as reduction in HETE formationMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Human)
Friedrich-Schiller-University
Curated by ChEMBL
Friedrich-Schiller-University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human recombinant 5-LO assessed as product formation by cell-free assay relative to controlMore data for this Ligand-Target Pair
TargetDual specificity protein kinase CLK1(Human)
Institut De Chimie Des Substances Naturelles
Curated by ChEMBL
Institut De Chimie Des Substances Naturelles
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CLK1 (unknown origin) assessed as inhibition of phosphate incorporation into substrate in presence of radiolabeled ATPMore data for this Ligand-Target Pair
TargetCDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7(Human)
Palack£
Curated by ChEMBL
Palack£
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of GST-tagged CDK7/cyclinH/MAT1 (unknown origin) expressed in Baculovirus infected Sf9 cells using YSPTSPS-2 KK peptide as substrate as su...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of JAK2 (unknown origin)More data for this Ligand-Target Pair