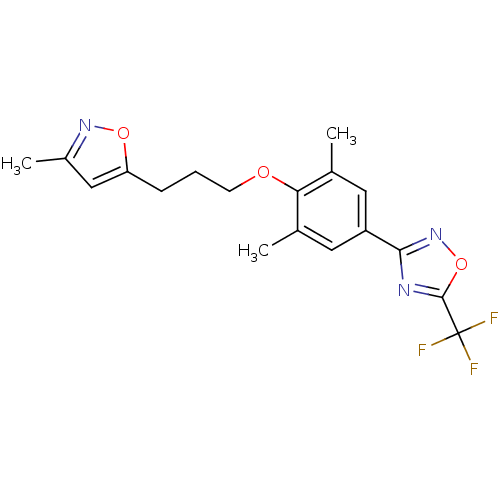
BDBM50111469 3-(3,5-dimethyl-4-(3-(3-methylisoxazol-5-yl)propoxy)phenyl)-5-(trifluoromethyl)-1,2,4-oxadiazole::3-{3,5-Dimethyl-4-[3-(3-methyl-isoxazol-5-yl)-propoxy]-phenyl}-5-trifluoromethyl-[1,2,4]oxadiazole::3-{3,5-Dimethyl-4-[3-(3-methyl-isoxazol-5-yl)-propoxy]-phenyl}-5-trifluoromethyl-[1,2,4]oxadiazole(Pleconaril)::5-[3-[2,6-dimethyl-4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]phenoxy]propyl]-3-methylisoxazole::CHEMBL29609::Pleconaril::Pleconaril3-{3,5-Dimethyl-4-[3-(3-methyl-isoxazol-5-yl)-propoxy]-phenyl}-5-trifluoromethyl-[1,2,4]oxadiazole
SMILES Cc1cc(CCCOc2c(C)cc(cc2C)-c2noc(n2)C(F)(F)F)on1
InChI Key InChIKey=KQOXLKOJHVFTRN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 50111469
Found 23 hits for monomerid = 50111469
Affinity DataEC50: 4nMAssay Description:Inhibition of Rhinovirus A89 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Inhibition of Rhinovirus A85 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Inhibition of Rhinovirus A28 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Inhibition of Rhinovirus A02 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Inhibition of Rhinovirus B14 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 58nMAssay Description:Inhibition of HRV Protease 3CP (serotype 14).More data for this Ligand-Target Pair
Affinity DataEC50: 167nMAssay Description:Inhibition of Rhinovirus B70 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataEC50: 251nMAssay Description:Activation of human PXR expressed in african green monkey CV1 cells transfected with pSG5-hPXRDATG and (ER6)3-tk-CAT reporterMore data for this Ligand-Target Pair
Affinity DataEC50: 490nMAssay Description:Inhibition of Rhinovirus A08 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.31E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase alpha(Human)
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant full length GST tagged P14KIII alpha (residues 1 to 854) incubated for 10 mins by ADP-Glo kinase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Human)
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Korea Research Institute of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human recombinant GST tagged P14KIII beta incubated for 10 mins by ADP-Glo kinase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Time dependent inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Time dependent inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Time dependent inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Time dependent inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMAssay Description:Time dependent inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Inhibition of Enterovirus 71 Shenzhen/120F1/09 capsid infected in human RD cells assessed as reduction in virus-induced cell death after 72 hrs by CC...More data for this Ligand-Target Pair
Affinity DataEC50: >1.28E+5nMAssay Description:Inhibition of Rhinovirus B42 capsid infected in human HeLa Rh cells assessed as reduction in virus-induced cytopathic effect after 3 days by MTS assa...More data for this Ligand-Target Pair