 Found 18 hits for monomerid = 50101858
Found 18 hits for monomerid = 50101858 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Human) | BDBM50101858
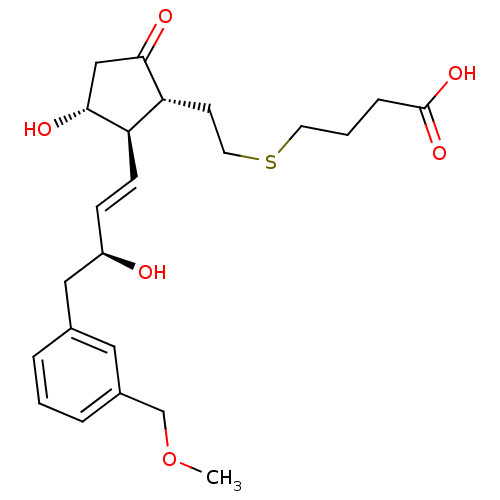
(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 0.142 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Human) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Human) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Rat) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Human) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | n/a | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Human) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 0.396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Human) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mouse) | BDBM50101858

(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | GoogleScholar
| UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data