

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50199189 6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-dione 5'-triphosphate::CHEMBL413841
SMILES: Cc1cc(=O)n([C@@H]2O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)[nH]1
InChI Key: InChIKey=AROARRWARGPJJC-UHFFFAOYSA-N
Data: 3 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 4 (Human) | BDBM50199189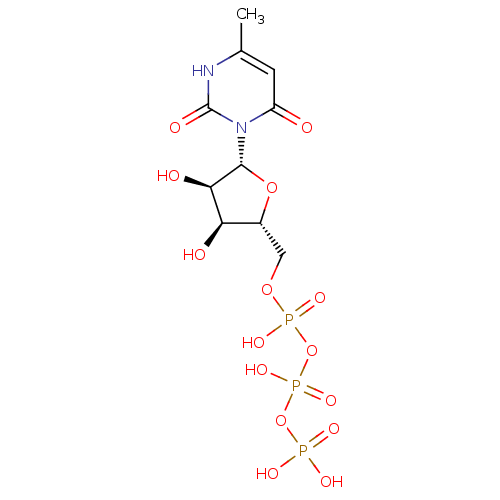 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | GoogleScholar | UniChem | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Human) | BDBM50199189 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | GoogleScholar | UniChem | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Human) | BDBM50199189 (6-methyl-3-beta-D-ribofuranosyl(1H)pyrimidine-2,4-...) | GoogleScholar | UniChem | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||