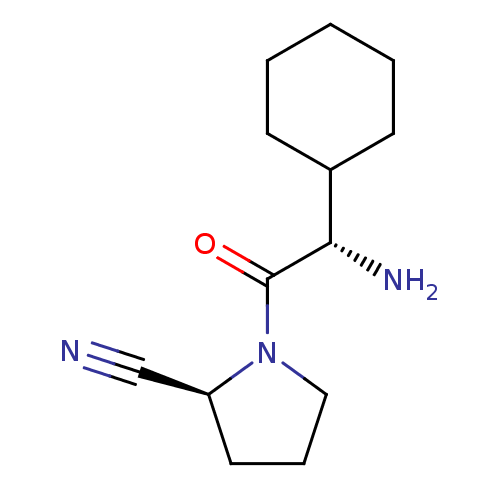
BDBM11694 (2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidine-2-carbonitrile::BMCL15687 Compound 3::CHEMBL307636::Cyclohexylglycine-(2S)-cyanopyrolidine 3::Cyclohexylglycine-(2S)-cyanopyrrolidine 2
SMILES N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C#N
InChI Key InChIKey=SXNUNNAPZNTPQV-RYUDHWBXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 11694
Found 21 hits for monomerid = 11694
Affinity DataKi: 1.40nMAssay Description:In vitro test for inhibitory activity against human dipeptidyl peptidase IV.More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Binding affinity towards Dipeptidyl peptidase IV (DPP-IV)More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 3.99E+4nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from rat plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from Caco-2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:In vitro inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) extracted from human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:In vitro inhibitory activity against Dipeptidyl-peptidase II (DPP-II) extracted from bovine kidney homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from rat plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+5nMAssay Description:In vitro inhibitory activity against Dipeptidyl peptidase II (DPP II) obtained form bovine kidney homogenateMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMAssay Description:In vitro inhibitory activity against Post-proline cleaving enzyme (PPCE) obtained from human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.15nMAssay Description:Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibitory concentration against Dipeptidyl-peptidase 8More data for this Ligand-Target Pair
Affinity DataIC50: 3.99E+4nMAssay Description:Inhibitory concentration against DPP-II [Quiescent cell proline dipeptidase] or DPP-VIIMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory concentration against Dipeptidyl-peptidase IV [DPP-IV]More data for this Ligand-Target Pair
Affinity DataIC50: 12nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 3.99E+4nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair