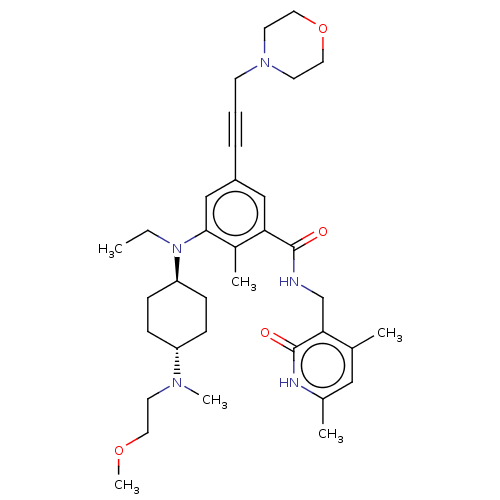
BDBM155254 US10098888, Compound 2::US9006242, 2
SMILES CCN([C@H]1CC[C@@H](CC1)N(C)CCOC)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CCN1CCOCC1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 155254
Found 15 hits for monomerid = 155254
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affinity DataKi: <3nMAssay Description:Inhibition of EZH2 (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.4Assay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.4Assay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.4Assay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:General Materials. S-adenosylmethionine (SAM), S-adenosylhomocyteine (SAH), bicine, KCI, Tween20, dimethylsulfoxide (DMSO) and bovine skin gelatin (B...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:General Materials. S-adenosylmethionine (SAM), S-adenosylhomocyteine (SAH), bicine, KCI, Tween20, dimethylsulfoxide (DMSO) and bovine skin gelatin (B...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >100nMAssay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of EZH2 Y641F mutant (unknown origin)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Homo sapiens (Human))
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affiliated Hospital Of Guangdong Medical University
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of EZH2 (unknown origin) assessed as reduction in H3K27me3 levelMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.4Assay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMpH: 7.4Assay Description:The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso...More data for this Ligand-Target Pair