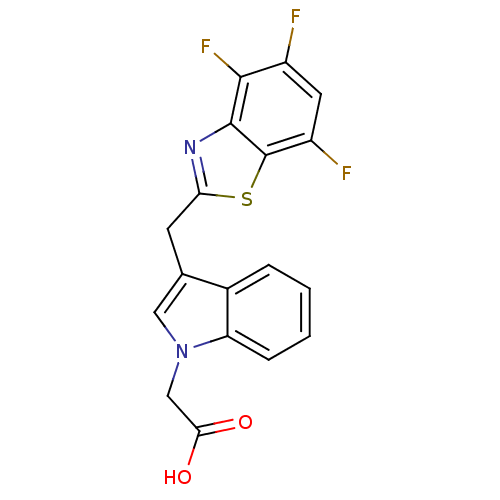
BDBM16469 2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methyl]-1H-indol-1-yl}acetic acid::CHEMBL363387::IDD-676::Indoleacetic Acid Inhibitor 9::Lidorestat::{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methyl]-1H-indol-1-yl}acetic acid
SMILES OC(=O)Cn1cc(Cc2nc3c(F)c(F)cc(F)c3s2)c2ccccc12
InChI Key InChIKey=KYHVTMFADJNSGS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 16469
Found 5 hits for monomerid = 16469
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute For Diabetes Discovery
The Institute For Diabetes Discovery
Affinity DataIC50: 5nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member A1(Homo sapiens (Human))
The Institute For Diabetes Discovery
The Institute For Diabetes Discovery
Affinity DataIC50: 2.70E+4nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute For Diabetes Discovery
The Institute For Diabetes Discovery
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of human recombinant aldose reductase 1 after 10 mins by spectrophotometry analysisMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute For Diabetes Discovery
The Institute For Diabetes Discovery
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Slovak Academy Of Sciences
Curated by ChEMBL
Slovak Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of Wistar rat lens aldose reductase using D,L-glyceraldehyde as substrate incubated for 1 min measured for 4 mins by spectrophotometryMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)