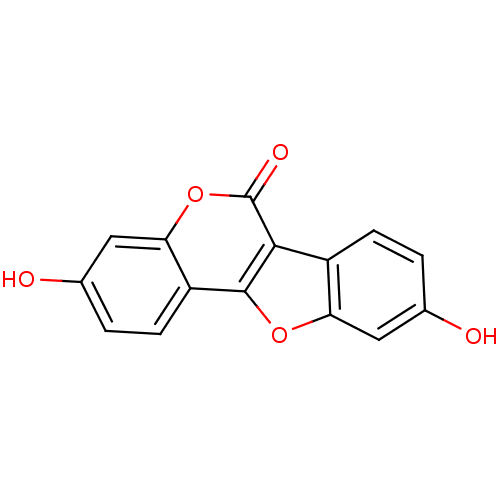
BDBM23451 5,14-dihydroxy-8,17-dioxatetracyclo[8.7.0.0^{2,7}.0^{11,16}]heptadeca-1(10),2,4,6,11(16),12,14-heptaen-9-one::7,12-Dihydroxycoumestan::CHEMBL30707::Chrysanthin::Coumestrol::Cumoestrol::US8552057, 3
SMILES c1cc2c(cc1O)oc-3c2C(=O)Oc4c3ccc(c4)O
InChI Key InChIKey=ZZIALNLLNHEQPJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 23451
Found 15 hits for monomerid = 23451
Affinity DataIC50: 2nMAssay Description:Binding affinity against human estrogen receptor beta (ER beta) in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Binding affinity against human estrogen receptor alpha in competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 18.6nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 18.6nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 18.6nMAssay Description:The binding affinity and selectivity of candidate molecules yielded from database screening were determined by a fluorescent polarization competitive...More data for this Ligand-Target Pair
Affinity DataIC50: 75.7nMAssay Description:Binding affinity for human Estrogen receptor AlphaMore data for this Ligand-Target Pair
Affinity DataIC50: 75.7nMAssay Description:The binding affinity and selectivity of candidate molecules yielded from database screening were determined by a fluorescent polarization competitive...More data for this Ligand-Target Pair
Affinity DataIC50: 75.7nMAssay Description:Binding affinity for human Estrogen receptor AlphaMore data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha 3(Human)
University of Padua and Institute of Biomolecular Chemistry
Curated by ChEMBL
University of Padua and Institute of Biomolecular Chemistry
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human CK2 using HRRRDDD-SDDD-NH2 as substrate after 30 mins by ADP-Glo reagent based luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Alpha-glucosidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 7.67E+3nMAssay Description:Inhibition of CK2alpha (unknown origin)More data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha 3(Human)
University of Padua and Institute of Biomolecular Chemistry
Curated by ChEMBL
University of Padua and Institute of Biomolecular Chemistry
Curated by ChEMBL
Affinity DataKi: 7.67E+3nMAssay Description:Inhibition of CK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Antagonist activity at human PXR transfected in African green monkey CV1 cells assessed as inhibition of SR12813-induced transactivation after 24 hrs...More data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Wilson-Smith/1933 H1N1...)
Texas Southern University
Curated by ChEMBL
Texas Southern University
Curated by ChEMBL
Affinity DataIC50: 3.78E+4nMAssay Description:Inhibition of Influenza A virus H5N1 neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.27E+4nMAssay Description:Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881)More data for this Ligand-Target Pair
Activity Spreadsheet -- ITC Data from BindingDB
 Found 1 hit for monomerid = 23451
Found 1 hit for monomerid = 23451
ITC DataΔG°: -6.56kcal/mole −TΔS°: -4.88kcal/mole ΔH°: -1.68kcal/mole logk: 5.49E+4
pH: 7.0 T: 30.00°C
pH: 7.0 T: 30.00°C
 3D Structure (crystal)
3D Structure (crystal)